Contrast-Enhanced Ultrasound with Perfluorobutane for Hepatocellular Cancer Surveillance: Our Initial Local Experience
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2024 Mar;27(1):e16-27 | Epub 20 March 2024
Contrast-Enhanced Ultrasound with Perfluorobutane for Hepatocellular Cancer Surveillance: Our Initial Local Experience
YS Chan1,2, CCM Cho1,2, TY Yuen1,2, HY Hung1,2, PKM Wong1, MMY Sou1, SSY Ho2, GLH Wong3, WCW Chu2
1 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR, China
2 Department of Imaging and Interventional Radiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
3 Medical Data Analytic Centre, Department of Medicine and Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
Correspondence: Dr CM Cho, Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR,
China. Email: ccm193@ha.org.hk
Submitted: 11 October 2022; Accepted: 20 March 2023.
Contributors: CCMC, SSYH, GLHW and WCWC designed the study. CCMC, TYY, HYH, PKMW and MMYS acquired the data. YSC and PKMW analysed the data. YSC drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: GLHW has served as an advisory committee member for Gilead Sciences and Janssen, as a speaker for Abbot, AbbVie, Bristol Myers Squibb, Echosens, Furui, Gilead Sciences, Janssen and Roche, and received research grant from Gilead Sciences. As an editor of the journal, WCWC was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The research was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (Ref No.: 2018.542) and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice. All patients gave informed consent for all treatments and procedures, and consent for publication.
Abstract
Introduction
Ultrasound is the most commonly used modality for hepatocellular cancer (HCC) surveillance in Hong Kong but has limitations in lesion characterisation. A second-generation perfluorobutane (PFB) ultrasound contrast agent allows for lesion characterisation through the usual vascular enhancement phases and provides an additional late Kupffer phase. We reviewed current evidence of PFB use in HCC care and investigated the value of contrast-enhanced ultrasound with PFB (PFB-CEUS) compared with brightness mode (B-mode) ultrasound in surveillance for HCC in high-risk patients in Hong Kong.
Methods
This prospective single-centre study assessed 50 high-risk patients under HCC surveillance undergoing B-mode ultrasound and PFB-CEUS, followed by gadoxetic acid–enhanced magnetic resonance imaging (MRI) within 3 weeks of the initial ultrasound scan. The MRI findings were considered the reference standard for the diagnosis of HCC. Detection rates of all and small (≤ 2 cm) HCCs on both modalities and the adverse event rate for each modality were evaluated.
Results
The detection rate of small HCCs was 4% by B-mode ultrasound and 6% by PFB-CEUS. A total of four small HCCs were identified in our cohort. The immediate (day 0), short-term (day 7), and long-term (day 90) adverse event rates were 0%, 12% and 6%, respectively. All adverse events were mild and self-limiting, with an uncertain causal relationship to PFB administration.
Conclusion
PFB-CEUS is emerging as a useful imaging modality in evaluation of liver lesions and HCC detection. Our initial local experience provides positive agreement with the literature and identifies areas requiring further investigation.
Key Words: Carcinoma, hepatocellular; Diagnostic imaging; Liver neoplasms
中文摘要
以全氟丁烷對比增強超聲波監測肝細胞癌:我們的初步本地經驗
陳奕璇、曹子文、袁子祐、洪曉義、王嘉文、蘇妙怡、何倩儀、黃麗虹、朱昭穎
引言
超聲波是香港最常使用的肝細胞癌監測方式,但在病變定性方面有其局限性。第二代全氟丁烷超聲波造影劑可通過通常的血管增強期來表徵病變,且提供額外的更晚的Kupffer期。我們回顧了目前全氟丁烷在肝細胞癌臨床處理的證據,並調查全氟丁烷對比增強超聲波與亮度模式(B模式)超聲波在監測本港肝細胞癌高風險患者方面的價值。
方法
本前瞻性單一中心研究評估了50名接受肝細胞癌監測的高風險患者,他們接受了B模式超聲波和全氟丁烷對比增強超聲波掃描,隨後在初次超聲波掃描後3週內進行了釓塞酸增強磁力共振。磁力共振結果是診斷肝細胞癌的參考標準。我們評估了兩種模式下所有肝細胞癌和小肝細胞癌(≤ 2厘米)的檢出率以及不良事件發生率。
結果
B模式超聲波的小肝細胞癌檢出率為4%,全氟丁烷對比增強超聲波則為6%。我們的隊列中總共發現四宗小肝細胞癌。即時(第0天)、短期(第7天)和長期(第90天)不良事件發生率分別為0%、12%和6%。所有不良事件均為輕微且具有自限性,與全氟丁烷給藥之間的因果關係不確定。
結論
全氟丁烷對比增強超聲波正成為評估肝臟病變和肝細胞癌檢測的一種有用影像手段。我們最初的本地經驗與文獻一致,並確定了需要進一步研究的方向。
INTRODUCTION
According to the Hong Kong Cancer Registry,
hepatocellular carcinoma (HCC) is currently the fifth
leading cancer and third leading cause of cancer deaths
in Hong Kong despite the declining incidence of
hepatitis B infection in recent years.[1] Chronic hepatitis
B infection has an established association with, and
is the major cause of, HCC. The latest territory-wide
study performed in 2015 to 2016 showed a prevalence
of 7.8% for hepatitis B infection in Hong Kong.[2]
Many individuals with known infection are in imaging
surveillance programmes, mostly using ultrasound as a
screening tool.
Ultrasound is a relatively simple, radiation-free, widely available, low-cost imaging procedure with good patient
acceptance. Ultrasound alone, however, is of limited
sensitivity and is inadequate for an imaging diagnosis
of HCC, which relies on the characteristic enhancement
pattern of the lesion.[3]
Basis of Ultrasound Contrast Agents and
Technique of Contrast-Enhanced Ultrasound
The use of contrast-enhanced ultrasound dates back
to 1969, when agitated normal saline was used in
echocardiography, progressing to the wider application of manufactured microbubbles in the 2000s to image
different organs. Microbubbles are formed from an
inert gas protected by an outer shell and are smaller
than red blood cells. After intravenous injection, they
can pass through the pulmonary capillaries and enter
the systemic circulation. Being mainly extracellular
blood pool agents, they stay in the vasculature, unlike
iodinated contrast for computed tomography (CT) which
is a soluble agent that will pass through vessel walls and
can enter hepatocytes and the nephron. Both are able
to provide information about the vascular pattern of
the lesion of concern. The mechanism of action is that
microbubbles increase the backscatter of ultrasound
signal. They oscillate under ultrasound and the non-linear
oscillations can cause harmonic emissions. These signals
can be captured and processed to produce images with
enhanced differentiation between vascular structures
(with microbubbles within) and adjacent soft tissue.[4]
Currently, second-generation ultrasound contrast agents
(UCAs) are in use worldwide. Commonly known
second-generation UCAs include sulphur hexafluoride,
which is used in Hong Kong, and perfluorobutane (PFB)
which has been licensed in multiple countries for liver-specific
imaging. Sonazoid (GE Healthcare, Milwaukee
[WI], US) essentially contains PFB microbubbles stabilised with a lipid coating with a well-defined
diameter of approximately 3 μm. Sonazoid to be used in
imaging is prepared by reconstitution with 2-mL sterile
water for injection. The usual recommended dose was
0.015 mL/kg. In addition to the standard vascular phases
including arterial, portal venous and delayed phases, PFB
has the unique ability to be taken up by the Kupffer cells.
This enables Kupffer phase imaging which resembles the
imaging obtained with a nuclear hepatobiliary scan. The
microbubbles can stay in the Kupffer cells for up to a few
hours. This aids in lesion detection and characterisation.
During the Kupffer phase, the liver parenchyma should
normally be uniformly enhancing; the presence of defects
would indicate lesions devoid of Kupffer cells, allowing
otherwise subtle lesions, in particular, lesions that may
be isoechoic on brightness mode (B-mode) ultrasound, to
become visible. Most malignancies, hepatic or metastatic,
do not contain Kupffer cells, whereas benign entities such
as focal nodular hyperplasia do contain such cells. This
is comparable with using a liver-specific contrast agent
such as gadoxetic acid in magnetic resonance imaging
(MRI) for hepatobiliary phase imaging. Leveraging this
unique property of PFB, an imaging technique called
defect reperfusion imaging was developed, using an
additional contrast bolus injection for evaluation of the
vascular characteristics of a defect in the Kupffer phase,
further improving its diagnostic value.[5]
The time frames for the phases are as follows (assuming
a normal cardiac output): the arterial phase starts at 10
seconds, peaks at 30 to 50 seconds, and is sustained until
approximately 1 minute; the portovenous phase starts at
30 seconds, peaks at 80 to 90 seconds, and is sustained
until approximately 2 minutes; the late vascular phase
then ensues and is followed by the Kupffer phase
(also called the post-vascular phase), which starts at
approximately 10 minutes.[6]
Perfluorobutane Applications
In 2008, the superiority of contrast-enhanced ultrasound
with PFB (PFB-CEUS) versus unenhanced ultrasound
in the diagnosis of focal liver lesions was confirmed
by a phase III clinical trial by Miyamoto et al.[7] One
study reported an even higher sensitivity and accuracy
for PFB-CEUS than contrast-enhanced CT in detecting
hepatic malignancy.[8] CEUS has value for lesions that
are indeterminate on CT or MRI due to its heightened
sensitivity in detecting vascular enhancement and real-time
continuous evaluation of the enhancement pattern.[9]
Compared with the other widely applied second-generation
UCAs, several studies have confirmed the non-inferiority of PFB.[10] [11] A retrospective study
published in 2010 by Kan et al[12] found a high sensitivity
and specificity for detection of small (≤ 2 cm) HCCs with
additional tumours detected by PFB-CEUS compared
with contrast-enhanced CT.
Beyond aiding initial imaging diagnosis, PFB has been
found to increase the localisation rate of focal hepatic
lesions for percutaneous biopsy.[13] [14] Along the same
lines, lesion localisation for radiofrequency ablation can
also be improved, with a higher success rate and fewer
treatment sessions required.[15] [16] [17]
Several studies have explored the utility of PFB in
prediction of treatment response of HCC treated with
transarterial chemoembolisation and other targeted
therapies, using a reduction in lesion vascularity in an
early post-intervention scan to predict the outcome.[18] [19] [20] [21]
In 2021, Funaoka et al[22] published their retrospective
study testing the ability of PFB to evaluate the efficacy
of radiotherapy for HCC, which further expands the
potential indications of PFB-CEUS as the results were
encouraging.
Standardisation and Regulation
With the increasing recognition of PFB’s utility, a
consensus statement with guidelines for PFB’s use
was released in 2020 by the Asian Federation of
Societies for Ultrasound in Medicine and Biology.[6] In
the same year, the World Federation for Ultrasound in
Medicine and Biology also published a good practice
recommendation.[9] Part of the reporting standardisation of
liver imaging is reliant on the use of the Liver Reporting
and Data System (LI-RADS). The current version of
LI-RADS, however, only applies to CEUS performed
with sulphur hexafluoride microbubbles (SonoVue) and
lipid-coated perfluoropropane microbubbles (Definity).
An early study of a modified CEUS LI-RADS for PFB
showed the LI-RADS categories LR-5 and LR-M to be
good predictors of HCC and non-HCC malignancies.[23]
A recent study also found no statistically significant
difference between modified CEUS LI-RADS, CT, and
the 2018 version of MRI LI-RADS in 31 histologically
proven lesions.[24] Further inclusion of PFB is to be
expected in the next version of CEUS LI-RADS, which
can provide formal recognition of its value in HCC
imaging.[25]
Safety Consideration
PFB has an established low-risk profile. The incidence
of adverse events was quoted to be 0.5% to 11.4%.[6] [26] [27] No serious adverse events have been reported to date.[6]
Common adverse effects include diarrhoea, proteinuria,
and headache.[6] The overwhelming majority of these
reported events did not require treatment.[6] [26] [27] Urticaria
has been reported as an adverse effect. It must be noted
that despite the lack of reported cases, anaphylaxis is a
possible risk for any injectable agent. The most likely
culprit for this potential allergic reaction is the lipid shell
of PFB, which is derived from eggs. Therefore, egg
allergy is the one and only contraindication to PFB use.
Patients with renal insufficiency, or iodine or gadolinium
contrast allergy, can undergo PFB-CEUS.
Initial Local Experience
As PFB is an unregistered drug in Hong Kong, it is
not currently in widespread use. We performed a pilot
study to investigate the value of PFB-CEUS compared
with conventional B-mode ultrasound for surveillance
detection of HCC in high-risk patients.
METHODS
Design and Setting
This was a prospective, single-centre, single-arm study
performed in Prince of Wales Hospital, a tertiary referral
centre with hepatology and hepatobiliary surgery
services available. The STARD (Standards for Reporting
of Diagnostic Accuracy Studies) 2015 guidelines were
used for reporting our results.
Patient Recruitment
Patients were recruited by convenience sampling
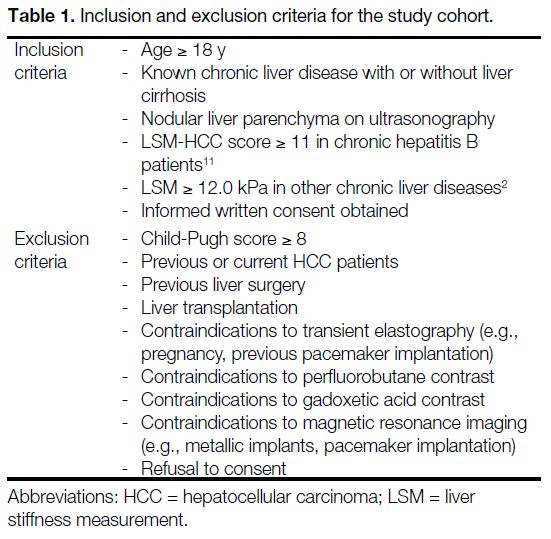
through clinician referral. A total of 50 patients were recruited from the period 1 June 2020 to 3 May 2021. The inclusion and exclusion criteria are listed in Table 1.
Table 1. Inclusion and exclusion criteria for the study cohort.
Imaging Workflow and Image Interpretation
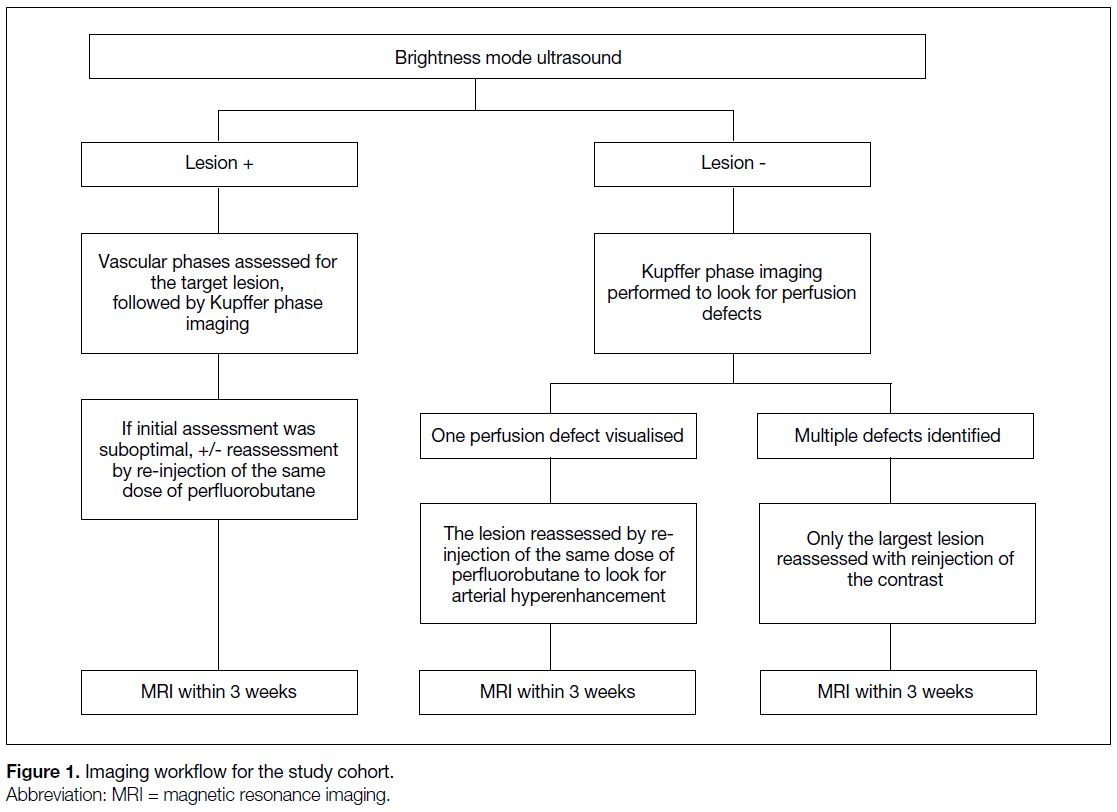
Eligible patients were assessed via a standard workflow,
starting with B-mode ultrasound and PFB-CEUS of the
liver performed with the same settings, followed by MRI
with gadoxetic acid (Primovist; Bayer Schering Pharma,
Berlin, Germany) within 3 weeks of the initial ultrasound
scan (Figure 1). The B-mode ultrasound and PFB-CEUS
were performed by two registered sonographers who
have > 20 years of ultrasound experience, following
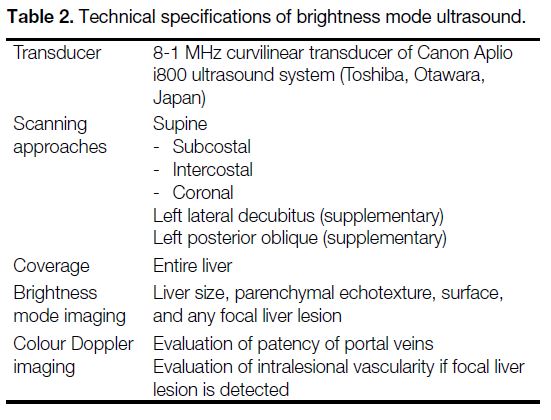
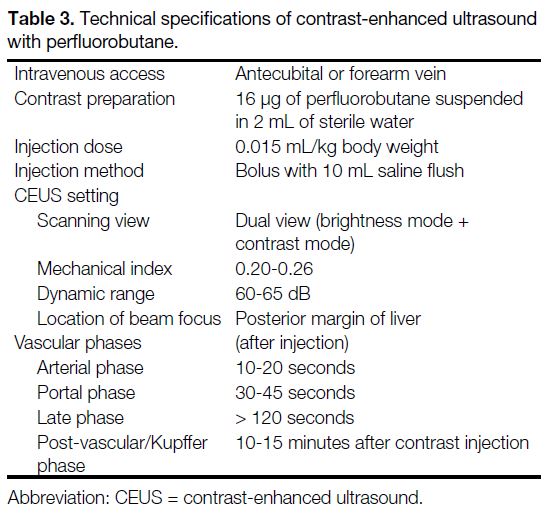
standard technical specifications (Tables 2 and 3).
Standard protocol was also implemented for the
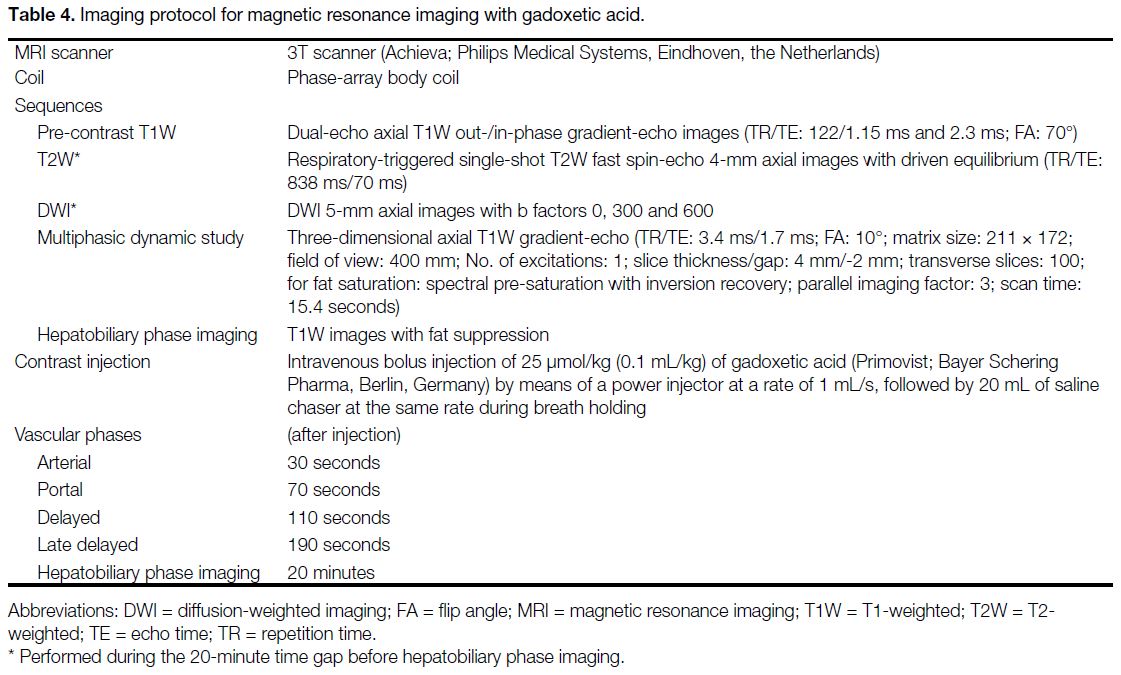
gadoxetic acid–enhanced MRI (Table 4). The assessors
were not blinded to clinical information of patients or
past radiological investigations.
Figure 1. Imaging workflow for the study cohort.
Table 2. Technical specifications of brightness mode ultrasound.
Table 3. Technical specifications of contrast-enhanced ultrasound with perfluorobutane.
Table 4. Imaging protocol for magnetic resonance imaging with gadoxetic acid.
The findings of B-mode ultrasound, PFB-CEUS,
and MRI were interpreted and reported separately by
three radiologists who had > 5 years of experience in
hepatobiliary imaging. As there were no standardised
interpretation criteria for B-mode or PFB-CEUS in the
diagnosis of HCC, interpretation of the findings from these
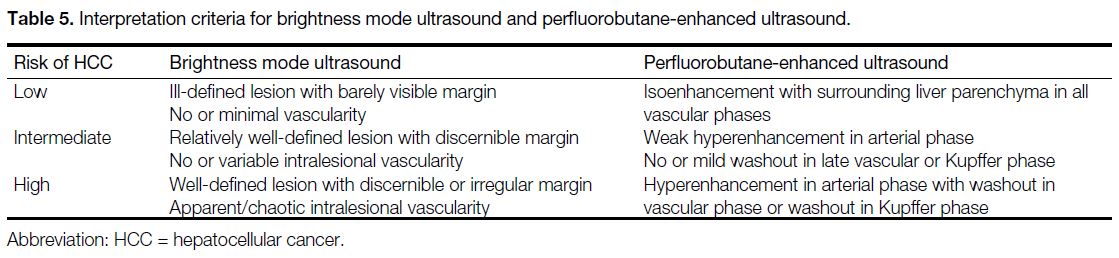
two scans were based on a 3-point Likert scale (Table 5),
while MRI findings were interpreted based on the 2018
version of LI-RADS.[28] The radiologists interpreting
MRI were blinded to the results of ultrasound and vice
versa. Any equivocal MRI findings were resolved by
consensus between two radiologists subspecialising in
hepatobiliary radiology. Those with negative findings on
both PFB-CEUS and MRI, meaning no focal lesion or
focal lesion(s) with low probability of HCC, followed
the standard surveillance programme. Patients with
intermediate probability for HCC were managed at the
discretion of the referring team and underwent closer
interval follow-up imaging or biopsy. Patients diagnosed
with HCC were referred to the Joint Hepatoma Clinic of
Prince of Wales Hospital for further management.
Table 5. Interpretation criteria for brightness mode ultrasound and perfluorobutane-enhanced ultrasound.
Reference Standards
For patients undergoing hepatic surgery or biopsy,
the final diagnosis was based on histological findings.
For those who did not undergo surgery or biopsy, the
diagnosis of HCC was based on MRI features fulfilling
the LR-5 or LR-4 category of the 2018 version of LI-RADS.[28] They were managed in a similar manner in our clinical practice. For LR-3 lesions, which were not
biopsied, additional follow-up CT or MRI was performed
at the discretion of the referring clinical team.
Primary Endpoints
The primary endpoints were the detection rate of small
(≤ 2 cm) HCCs on B-mode US and PFB-CEUS. The
detection rate was defined as the percentage of positive findings on the respective imaging modality confirmed
with a reference standard out of all patients in the study
cohort. A positive finding was defined as a high-risk
lesion on the Likert scale.
Secondary Endpoints
The secondary endpoints included the detection rate of
all sizes of HCC and immediate (day 0), short-term (day
7) and long-term (day 90) adverse event rates. Adverse
events were recorded according to the CTCAE (Common
Terminology Criteria for Adverse Events) version 5.0
with structured telephone interviews at 1 week and 90
days after the ultrasound examination.[29]
RESULTS
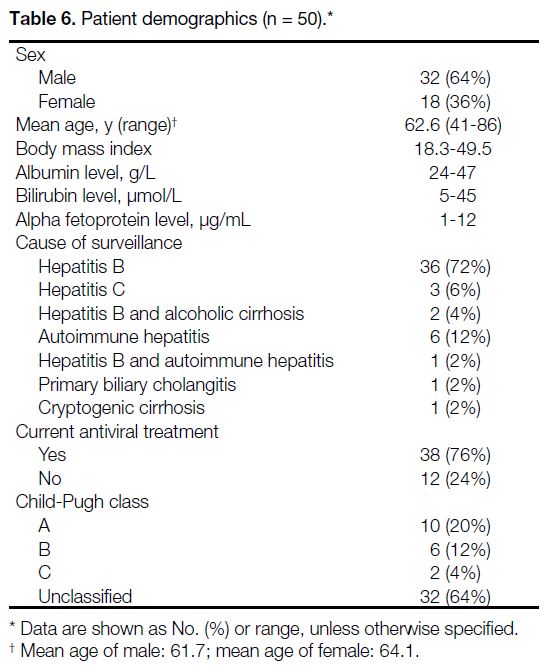
A total of 50 patients were included in the study,
consisting of 32 men and 18 women with a mean age of
62.6 years (Table 6). The majority (72%) of the patients were in the HCC surveillance programme due to hepatitis
B. All of the patients had had prior surveillance imaging.
For 94% of the patients, the previous surveillance scans
were B-mode ultrasound while the rest were contrast-enhanced
CT. The median time from prior surveillance
imaging to ultrasound evaluation our study was 12.5
months. The median time from PFB-CEUS to MRI was
11 days. None of the detected lesions underwent biopsy
or surgical resection during the study period.
Table 6. Patient demographics (n = 50).
Primary Endpoints
The detection rates of small HCCs were 4% (n = 2) by
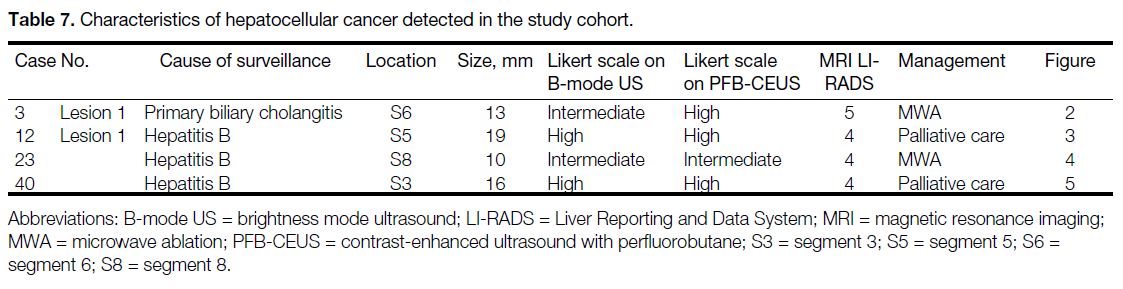
B-mode ultrasound and 6% (n = 3) by PFB-CEUS. A total of four small HCCs were identified in our cohort (Table 7). Figures 2, 3, 4, and 5 show the key imaging features of these four HCCs found in our cohort.
Table 7. Characteristics of hepatocellular cancer detected in the study cohort.
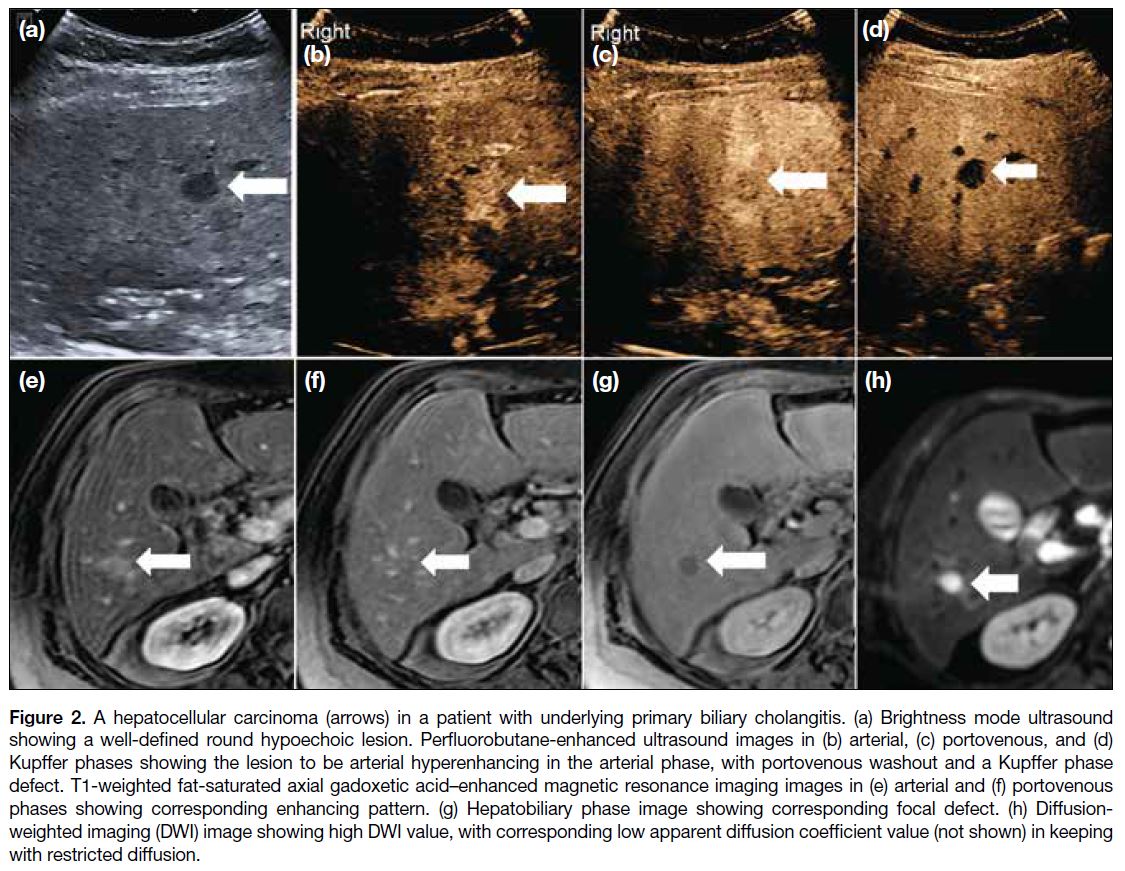
Figure 2. A hepatocellular carcinoma (arrows) in a patient with underlying primary biliary cholangitis. (a) Brightness mode ultrasound showing a well-defined round hypoechoic lesion. Perfluorobutane-enhanced ultrasound images in (b) arterial, (c) portovenous, and (d) Kupffer phases showing the lesion to be arterial hyperenhancing in the arterial phase, with portovenous washout and a Kupffer phase defect. T1-weighted fat-saturated axial gadoxetic acid–enhanced magnetic resonance imaging images in (e) arterial and (f) portovenous phases showing corresponding enhancing pattern. (g) Hepatobiliary phase image showing corresponding focal defect. (h) Diffusion-weighted imaging (DWI) image showing high DWI value, with corresponding low apparent diffusion coefficient value (not shown) in keeping with restricted diffusion.
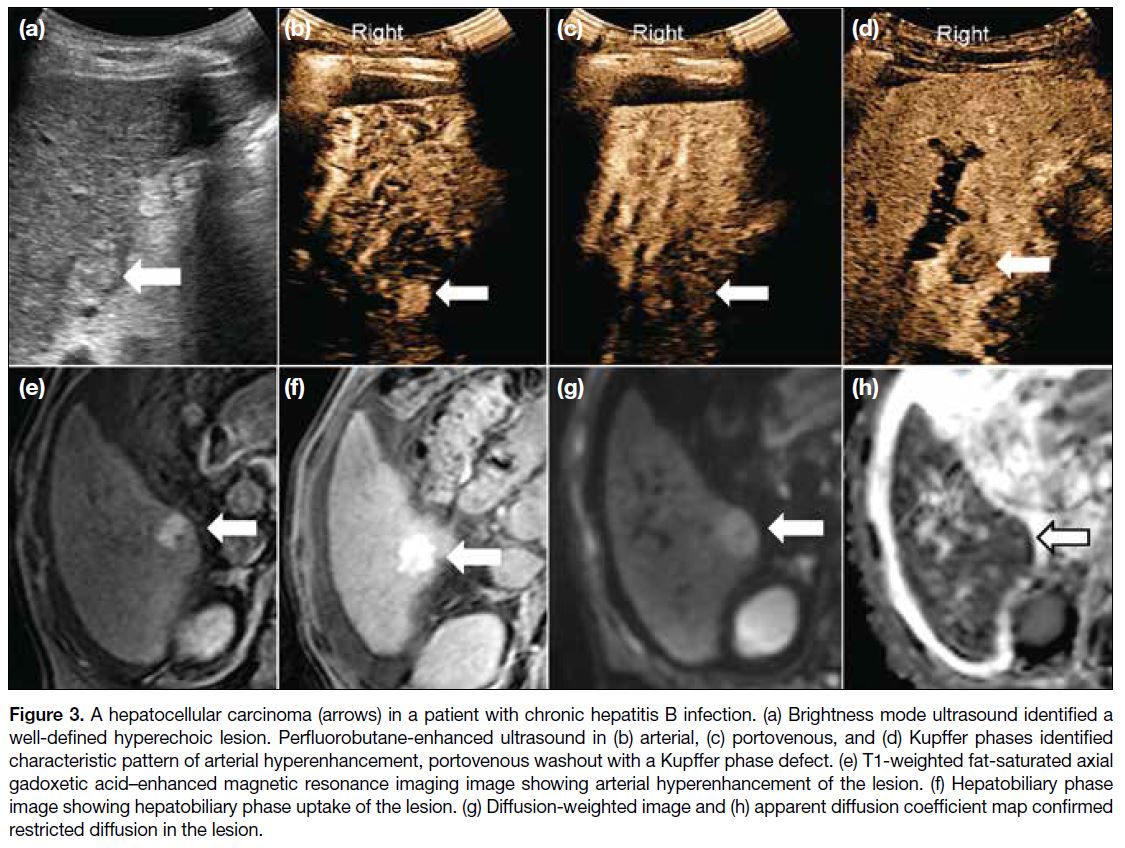
Figure 3. A hepatocellular carcinoma (arrows) in a patient with chronic hepatitis B infection. (a) Brightness mode ultrasound identified a
well-defined hyperechoic lesion. Perfluorobutane-enhanced ultrasound in (b) arterial, (c) portovenous, and (d) Kupffer phases identified
characteristic pattern of arterial hyperenhancement, portovenous washout with a Kupffer phase defect. (e) T1-weighted fat-saturated axial
gadoxetic acid–enhanced magnetic resonance imaging image showing arterial hyperenhancement of the lesion. (f) Hepatobiliary phase
image showing hepatobiliary phase uptake of the lesion. (g) Diffusion-weighted image and (h) apparent diffusion coefficient map confirmed
restricted diffusion in the lesion.
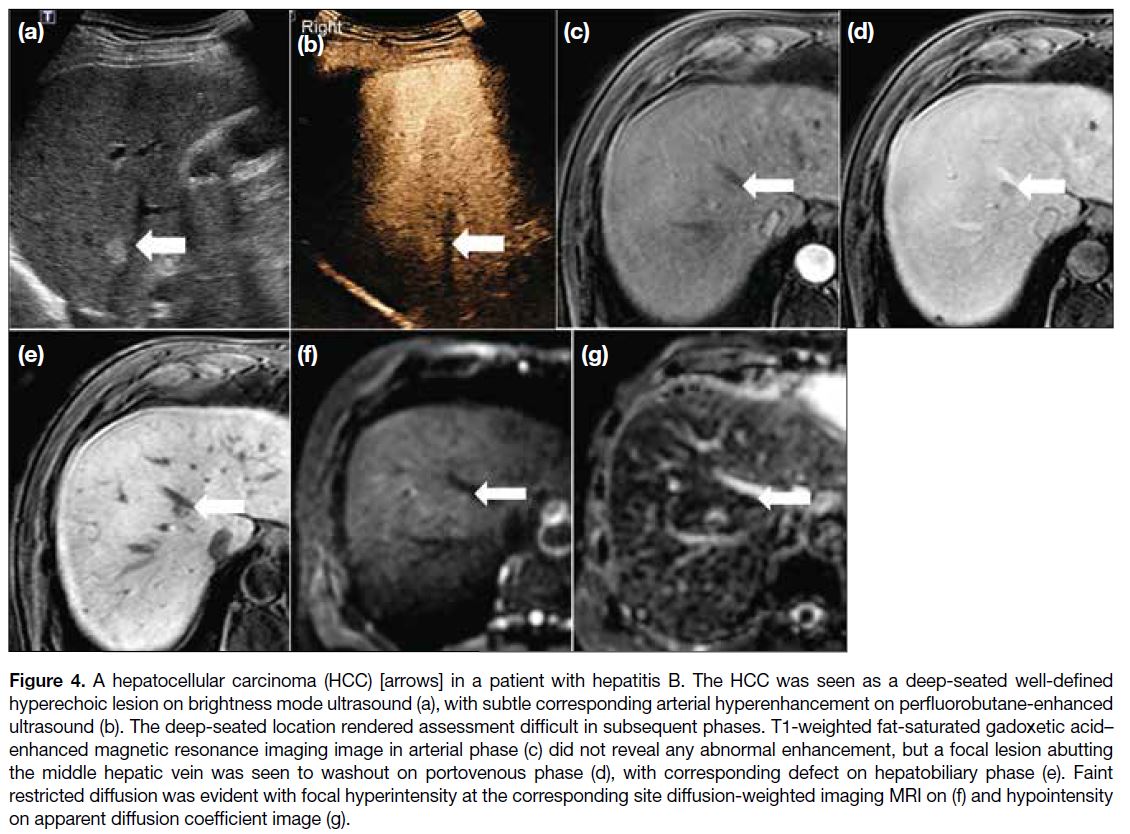
Figure 4. A hepatocellular carcinoma (HCC) [arrows] in a patient with hepatitis B. The HCC was seen as a deep-seated well-defined hyperechoic lesion on brightness mode ultrasound (a), with subtle corresponding arterial hyperenhancement on perfluorobutane-enhanced ultrasound (b). The deep-seated location rendered assessment difficult in subsequent phases. T1-weighted fat-saturated gadoxetic acid–enhanced magnetic resonance imaging image in arterial phase (c) did not reveal any abnormal enhancement, but a focal lesion abutting the middle hepatic vein was seen to washout on portovenous phase (d), with corresponding defect on hepatobiliary phase (e). Faint restricted diffusion was evident with focal hyperintensity at the corresponding site diffusion-weighted imaging MRI on (f) and hypointensity on apparent diffusion coefficient image (g).
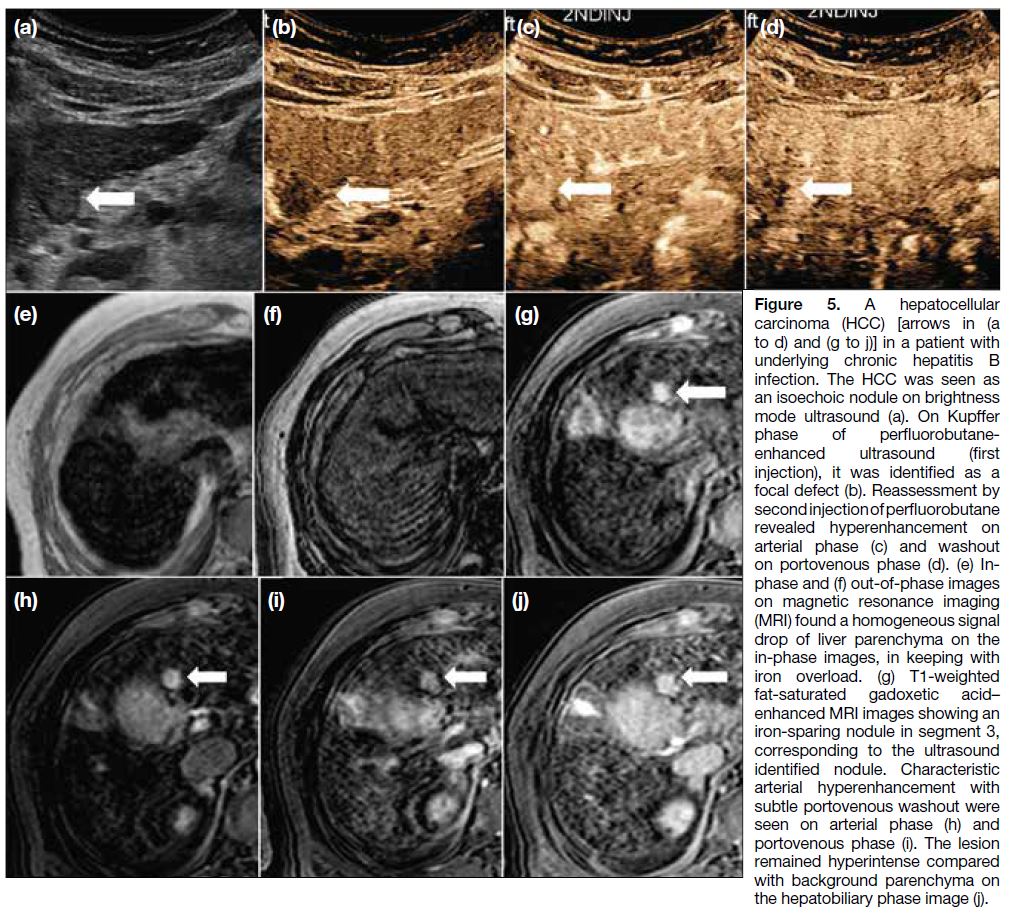
Figure 5. A hepatocellular
carcinoma (HCC) [arrows in (a to d) and (g to j)] in a patient with underlying chronic hepatitis B infection. The HCC was seen as an isoechoic nodule on brightness mode ultrasound (a). On Kupffer phase of perfluorobutane-enhanced ultrasound (first injection), it was identified as a focal defect (b). Reassessment by second injection of perfluorobutane revealed hyperenhancement on arterial phase (c) and washout on portovenous phase (d). (e) In-phase and (f) out-of-phase images on magnetic resonance imaging (MRI) found a homogeneous signal drop of liver parenchyma on the in-phase images, in keeping with iron overload. (g) T1-weighted fat-saturated gadoxetic acid–enhanced MRI images showing an iron-sparing nodule in segment 3, corresponding to the ultrasound identified nodule. Characteristic arterial hyperenhancement with subtle portovenous washout were seen on arterial phase (h) and portovenous phase (i). The lesion remained hyperintense compared with background parenchyma on the hepatobiliary phase image (j).
Secondary Endpoints
There was no HCC > 2 cm identified in the study cohort.
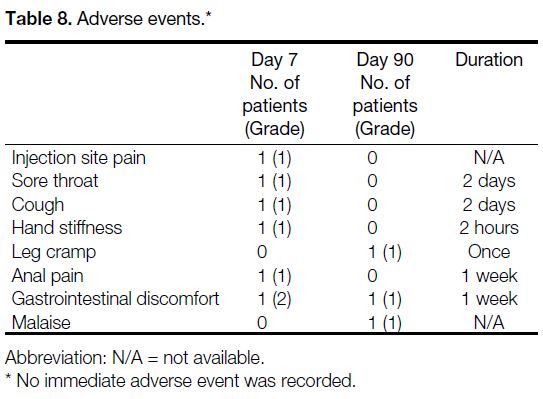
The immediate adverse event rate was 0%, the short-term
adverse event rate was 12% (n = 6), and the long-term
adverse event rate was 6% (n = 3) [Table 8]. All of
the adverse events were mild and self-limiting, with an
uncertain causal relationship to PFB administration.
Table 8. Adverse events.
DISCUSSION
In our study, PFB-CEUS had a slightly better detection rate of HCC than B-mode ultrasound. Although all of
the HCCs were detectable by either modality, B-mode
ultrasound falsely classified two of the lesions as
intermediate risk on the Likert scale, while PFB-CEUS
was able to add value by reclassifying one of these
lesions underclassified by B-mode ultrasound as a high-risk
lesion. This finding is in keeping with that of Park
et al,[30] though no statistical significance was identified in
their study for the detection of additional small HCCs by
PFB-CEUS.
With this initial trial of PFB application, we have
learned a few lessons in its use. First of all, deep lesions
are difficult to evaluate by PFB-CEUS. The one false-negative
lesion on PFB-CEUS was a lesion located deep
in the parenchyma, 9 cm from the skin surface (Figure 3). The difficulty of evaluating deep lesions by PFB-CEUS
was recognised due to ultrasound attenuation in
low mechanical index settings.[6] Although switching to
high mechanical index settings in B-mode ultrasound
can aid assessment, the limited assessment by PFB-CEUS
precludes its value addition in deep lesion
characterisation.
In addition to the technical aspect of PFB use, we made
an observation of a unique property. One of the LR-4
lesions was identified in the Kupffer phase as a defect
on PFB-CEUS, while showing hyperintensity on the
hepatobiliary phase in gadoxetic acid–enhanced MRI.
This evokes a complex discussion involving both PFB,
liver-specific MRI contrast properties and HCC cell
biology. PFB-CEUS has often been compared with
gadoxetic acid–enhanced MRI for its characteristic liver-specific
phase—the Kupffer phase. The two phases share
similarities by predominantly identifying HCC as focal
defects, but have fundamentally different principles
underlying them. For gadoxetic acid–enhanced MRI,
the hepatobiliary phase appearance is based upon the
organic anion transporting polypeptide 8 (OATP8) expression of cells. Normal hepatocytes take up contrast
through OATP, while both early and progressed HCCs
often have a reduced expression of OATP during
hepatocarcinogenesis, reducing their contrast uptake
in the hepatobiliary phase, leading to a hepatobiliary
phase defect.[31] Hepatobiliary phase–hyperintense HCC
is, however, not uncommon, constituting up to 10% of
cases.[32] It has been suggested that hepatobiliary phase
hyperintensity may be seen in some moderately- or well-differentiated
HCCs, but has been reported in poorly
differentiated HCC as well and is due to paradoxical
upregulation of OATP.[33] On the other hand, for PFB-CEUS,
the contrast between normal parenchyma and
HCC is achieved by differences in the number of Kupffer
cells. Kupffer cells are liver-specific macrophages,
which phagocytose the microbubbles, causing
enhancement of hepatic parenchyma. The Kupffer cell
count is seen to decline with hepatocarcinogenesis and has been suggested to decline more slowly than OATP8
expression.[32] The presence of a Kupffer phase defect
strongly suggests progression of HCC. Korenaga et al[34]
found that all the moderately and poorly differentiated
HCCs in their study showed Kupffer phase defects while
well-differentiated HCCs tended to lack them. Our LR-4
lesion did not undergo histological confirmation, but we
speculated that it is a moderately differentiated HCC
based on the uptake. Further studies are warranted to look
into the potential use of PFB-CEUS versus gadoxetic
acid–enhanced MRI for predicting the histological
differentiation of HCC.
We have also identified a potential advantage of using
PFB-CEUS, which is in patients with hepatic iron
overload. One of the patients was found to have heavy
iron deposition on MRI. This markedly impaired
the assessment on hepatobiliary phase as the whole liver remained hypointense due to iron deposition.
Iron overload is a recognised risk factor for HCC
development. Due to the paramagnetic effect of iron,
iron-overloaded liver parenchyma shows markedly
hypointense signal on T1-weighted and T2-weighted
images, providing excellent contrast for iron-sparing
HCC. It should be noted, however, that siderotic nodules
may also be found in such patients. Siderotic nodules are
hypointense, making them inconspicuous due to a similar
hypointense appearance to the surrounding liver tissue
which is also iron-overloaded. With the similar liver-specific
nature of PFB and liver-specific MRI contrast,
we pondered the possibility of the superiority of use of
PFB-CEUS in iron-overloaded liver in demonstrating
a liver-specific phase defect. Up to now, the effect of
hepatic iron overload on Kupffer cell function and
Kupffer phase defects for HCC detection has not been
described. Our study has identified a unique area in PFB-CEUS use that has not been previously described, and
further investigation into the use of PFB-CEUS Kupffer
phase imaging as an alternative screening method for
patients with hepatic iron overload should be carried
out. This also brings out the possibility of using PFB-CEUS
in other conditions where the hepatobiliary phase
assessment on MRI is limited, e.g., patients with poor
hepatic function, severe cirrhosis, or cholestasis.[30]
Limitation
The main limitation of our study is the small sample size and low incidence of HCC in our cohort, which limits the
potential for statistical evaluation. This study framework
served more as a standardised approach to our initial
experience with PFB use in HCC surveillance. Despite
the small size, we were able to identify a few points in
the use of PFB that require further development and
found additional value of PFB use that is in keeping with findings in the literature. Further studies with a larger
sample size may be attempted to allow for statistical
evaluation of this slowly maturing modality of choice.
CONCLUSION
PFB-CEUS is an emerging imaging modality in
evaluation of liver lesions and HCC detection, with
increased recognition worldwide and expanding
potential uses. Our initial local experience provides
positive agreement with the literature and identified areas
requiring further investigation, including correlation of
Kupffer phase defects and hepatobiliary phase defects
with histological differentiation, and potential use of
the Kupffer phase in assessment of patients with iron-overloaded
livers.
REFERENCES
1. Hospital Authority. Overview of Hong Kong Cancer Statistics of 2019. 2021. Available from: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202019.pdf. Accessed 27 Feb 2024.
2. Liu KS, Seto WK, Lau EH, Wong DK, Lam YF, Cheung KS, et al. A territorywide prevalence study on blood-borne and enteric viral hepatitis in Hong Kong. J Infect Dis. 2019;219:1924-33. Crossref
3. Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ann Intern Med. 2015;162:697-711. Crossref
4. Baun J. Contrast-enhanced ultrasound: a technology primer. J Diagn Med Sonogr. 2017;33:446-52. Crossref
5. Kudo M, Hatanaka K, Maekawa K. Newly developed novel
ultrasound technique, defect reperfusion ultrasound imaging,
using Sonazoid in the management of hepatocellular carcinoma.
Oncology. 2010;78 Suppl 1:40-5. Crossref
6. Lee JY, Minami Y, Choi BI, Lee WJ, Chou YH, Jeong WK, et al.
The AFSUMB consensus statements and recommendations for the
clinical practice of contrast-enhanced ultrasound using Sonazoid.
Ultrasonography. 2020;39:191-220. Crossref
7. Miyamoto Y, Ito T, Takada E, Omoto K, Hirai T, Moriyasu F.
Efficacy of Sonazoid (perflubutane) for contrast-enhanced
ultrasound in the differentiation of focal breast lesions: phase 3
multicenter clinical trial. AJR Am J Roentgenol. 2014;202:W400-7. Crossref
8. Hatanaka K, Kudo M, Minami Y, Maekawa K. Sonazoid-enhanced
ultrasonography for diagnosis of hepatic malignancies: comparison
with contrast-enhanced CT. Oncology. 2008;75 Suppl 1:42-7. Crossref
9. Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN,
Cantisani V, et al. Guidelines and good clinical practice
recommendations for contrast-enhanced ultrasound (CEUS) in
the liver—update 2020—WFUMB in cooperation with EFSUMB,
AFSUMB, AIUM, and FLAUS. Ultraschall Med. 2020;41:562-85. Crossref
10. Zhai HY, Liang P, Yu J, Cao F, Kuang M, Liu FY, et al. Comparison
of Sonazoid and SonoVue in the diagnosis of focal liver lesions: a
preliminary study. J Ultrasound Med. 2019;38:2417-25. Crossref
11. Lv K, Zhai H, Jiang Y, Liang P, Xu HX, Du L, et al. Prospective
assessment of diagnostic efficacy and safety of SonazoidTM and
SonoVue® ultrasound contrast agents in patients with focal liver
lesions. Abdom Radiol (NY). 2021;46:4647-59. Crossref
12. Kan M, Hiraoka A, Uehara T, Hidaka S, Ichiryu M, Nakahara H, et al. Evaluation of contrast-enhanced ultrasonography using
perfluorobutane (Sonazoid®) in patients with small hepatocellular
carcinoma: comparison with dynamic computed tomography.
Oncol Lett. 2010;1:485-8. Crossref
13. Eso Y, Takai A, Takeda H, Matsumoto T, Lee M, Inuzuka T, et al. Sonazoid-enhanced ultrasonography guidance improves the quality of pathological diagnosis in the biopsy of focal hepatic lesions. Eur J Gastroenterol Hepatol. 2016;28:1462-7. Crossref
14. Park HS, Kim YJ, Yu MH, Jung SI, Jeon HJ. Real-time contrast-enhanced sonographically guided biopsy or radiofrequency ablation
of focal liver lesions using perfluorobutane microbubbles (Sonazoid):
value of Kupffer-phase imaging. J Ultrasound Med. 2015;34:411-21. Crossref
15. Minami Y, Kudo M, Hatanaka K, Kitai S, Inoue T, Hagiwara S, et al. Radiofrequency ablation guided by contrast harmonic sonography using perfluorocarbon microbubbles (Sonazoid) for
hepatic malignancies: an initial experience. Liver Int. 2010;30:759-64. Crossref
16. Masuzaki R, Shiina S, Tateishi R, Yoshida H, Goto E, Sugioka Y, et al. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:759-64. Crossref
17. Dohmen T, Kataoka E, Yamada I, Miura K, Ohshima S, Shibuya T, et al. Efficacy of contrast-enhanced ultrasonography in radiofrequency ablation for hepatocellular carcinoma. Intern
Med. 2012;51:1-7. Crossref
18. Shiozawa K, Watanabe M, Kikuchi Y, Kudo T, Maruyama K, Sumino Y. Evaluation of sorafenib for hepatocellular carcinoma by contrast-enhanced ultrasonography: a pilot study. World J
Gastroenterol. 2012;18:5753-8. Crossref
19. Sugimoto K, Moriyasu F, Saito K, Rognin N, Kamiyama N, Furuichi Y, et al. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33:605-15. Crossref
20. Xia Y, Kudo M, Minami Y, Hatanaka K, Ueshima K, Chung H, et al. Response evaluation of transcatheter arterial chemoembolization in hepatocellular carcinomas: the usefulness of Sonazoid-enhanced harmonic sonography. Oncology. 2008;75 Suppl 1:99-105. Crossref
21. Kono Y, Lucidarme O, Choi SH, Rose SC, Hassanein TI, Alpert
E, et al. Contrast-enhanced ultrasound as a predictor of treatment
efficacy within 2 weeks after transarterial chemoembolization of
hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:57-65. Crossref
22. Funaoka A, Numata K, Takeda A, Saigusa Y, Tsurugai Y, Nihonmatsu H, et al. Use of contrast-enhanced ultrasound with Sonazoid for evaluating the radiotherapy efficacy for hepatocellular
carcinoma. Diagnostics (Basel). 2021;11:486. Crossref
23. Sugimoto K, Kakegawa T, Takahashi H, Tomita Y, Abe M, Yoshimasu Y, et al. Usefulness of modified CEUS LI-RADS for the diagnosis of hepatocellular carcinoma using Sonazoid. Diagnostics
(Basel). 2020;10:828. Crossref
24. Hwang JA, Jeong WK, Min JH, Kim YY, Heo NH, Lim HK. Sonazoid-enhanced ultrasonography: comparison with CT/MRI
liver imaging reporting and data system in patients with suspected
hepatocellular carcinoma. Ultrasonography. 2021;40:486-98. Crossref
25. Minami Y, Kudo M. Contrast-enhanced ultrasonography with Sonazoid in hepatocellular carcinoma diagnosis. Hepatoma Res. 2020;6:46. Crossref
26. Miyamoto Y, Ito T, Takada E, Omoto K, Hirai T, Moriyasu
F. Efficacy of Sonazoid (perflubutane) for contrast-enhanced
ultrasound in the differentiation of focal breast lesions: phase 3
multicenter clinical trial. AJR Am J Roentgenol. 2014;202:W400-7. Crossref
27. Mori Y, Hayakawa A, Abe K, Tanigawa M, Takahashi M, Naruse H, et al. The safety and the efficacy of ultrasound contrast media perflubutane microbubbles in clinical practice. Choonpa Igaku. 2011;38:541-48. Crossref
28. American College of Radiology Committee on LI-RADS. CT/MRI LI-RADS® v2018 CORE. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf. Accessed 17 Jul 2022.
29. Department of Health and Human Services, United States
Government. Common Terminology Criteria for Adverse Events
(CTCAE) Version 5.0. 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 2 May 2022.
30. Park JH, Park MS, Lee SJ, Jeong WK, Lee JY, Park MJ, et al.
Contrast-enhanced US with perfluorobutane for hepatocellular
carcinoma surveillance: a multicenter diagnostic trial (SCAN).
Radiology. 2019;292:638-46. Crossref
31. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273:30-50. Crossref
32. Kim MJ, Rhee HJ, Jeong HT. Hyperintense lesions on gadoxetate disodium–enhanced hepatobiliary phase imaging. AJR Am J Roentgenol. 2012;199:W575-86. Crossref
33. Kim JY, Kim MJ, Kim KA, Jeong HT, Park YN. Hyperintense HCC on hepatobiliary phase images of gadoxetic acid–enhanced MRI: correlation with clinical and pathological features. Eur J Radiol. 2012;81:3877-82. Crossref
34. Korenaga K, Korenaga M, Furukawa M, Yamasaki T, Sakaida I.
Usefulness of Sonazoid contrast-enhanced ultrasonography for
hepatocellular carcinoma: comparison with pathological diagnosis
and superparamagnetic iron oxide magnetic resonance images. J
Gastroenterol. 2009;44:733-41. Crossref