Tolerability and Efficacy of Palbociclib and Ribociclib in Breast Cancer in Hong Kong: A Single-Centre Study
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2024 Mar;27(1):e5-15 | Epub 13 March 2024
Tolerability and Efficacy of Palbociclib and Ribociclib in Breast Cancer in Hong Kong: A Single-Centre Study
JLC Hung, IS Soong
Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr JLC Hung, Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email:
Submitted: 6 April 2023; Accepted: 15 August 2023.
Contributors: Both authors designed the study. JLCH acquired and analysed the data, and drafted the manuscript. Both authors critically revised the manuscript for important intellectual content. Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, JLCH was not involved in the peer review process. ISS has disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Hong Kong East Cluster Research Ethics Committee of Hospital Authority, Hong Kong (Ref No.: HKECREC-2022-67). The requirement for informed patient consent was waived by the Committee due to the retrospective nature of the
study.
Supplementary Material: The supplementary material was provided by the authors and some information may not have been peer reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by the Hong Kong College of Radiologists. The Hong Kong College of Radiologists disclaims all liability and responsibility arising from any reliance placed on the content.
Abstract
Introduction
This study aimed to analyse the safety, tolerability, and other potential factors affecting the treatment outcome of advanced breast cancer (including inoperable stage III or stage IV, as per the eighth edition of the American Joint Committee on Cancer staging manual) treated with palbociclib or ribociclib at a single institution in Hong Kong.
Methods
The medical records of all breast cancer patients receiving palbociclib or ribociclib at a hospital in Hong Kong during the period of July 2016 to February 2022 were reviewed. Data regarding baseline demographics, treatment-related adverse events, need for dose reduction, and disease progression were collected.
Results
A total of 211 patients were included in the study, where 88.6% received palbociclib and 11.4% received ribociclib. Among the patients started on full doses (91.4% for palbociclib and 91.7% for ribociclib), 48.5% and 54.5% required dose reduction, respectively, most often due to neutropenia. No statistically significant factor could be identified for predicting the severity of neutropenia in this cohort. In patients on first-line treatment, dose reduction, treatment delay, high levels of oestrogen receptor and progesterone receptor were associated with longer progression-free survival, with respective p values of < 0.001, 0.010, 0.002, and 0.001.
Conclusion
Palbociclib and ribociclib were safe and well-tolerated in a predominantly Asian population in real-life
clinical practice, with comparable treatment outcomes to those quoted in international clinical trials. Dose reduction
did not compromise the treatment efficacy.
Key Words: Asian; Breast neoplasms; Drug tolerance; Hong Kong
中文摘要
在香港使用帕博西尼及瑞博西尼治療乳癌的耐受性及效用:單一中心研究
孔朗程、宋崧
引言
本研究旨在分析在香港某所醫院使用帕博西尼及瑞博西尼治療晚期乳癌(包括根據美國癌症聯合委員會癌症分期系統第8版分類的無法進行手術的第III期或第IV期)的安全性、耐受性及影響治療結果的其他潛在因素。
方法
我們回顧了2016年7月至2022年2月期間所有在香港某所醫院接受帕博西尼或瑞博西尼治療的乳癌患者的醫療紀錄,收集的資料包括基線人口特徵、與治療相關的不良事件、減少劑量的需要及病情惡化情況。
結果
本研究共包括211名患者,當中88.6%使用帕博西尼,11.4%使用瑞博西尼。在開始時使用全劑量的患者中(91.4%使用帕博西尼的患者及91.7%使用瑞博西尼的患者),分別有48.5%及54.5%需要減少劑量,大多由嗜中性白血球減少症引致。在預測本隊列的嗜中性白血球減少症的嚴重程度方面,我們找不到具統計學意義的因素。在接受一線治療的患者中,減少劑量、延遲治療、雌激素受體及孕酮受體水平偏高與較長的疾病無惡化存活相關,p值分別為< 0.001、0.010、0.002及0.001。
結論
在以亞裔人口為主的真實臨床診療情況中,帕博西尼及瑞博西尼是安全及具耐受性的藥物,其治療結果與多個國際臨床試驗所引述的相若。減少劑量並無降低治療效用。
INTRODUCTION
Breast cancer is the leading type of female cancer in
Hong Kong, accounting for 27.4% of female cancers
diagnosed in 2019,[1] of which approximately 70% to 80%
exhibit oestrogen receptor (ER) and/or progesterone
receptor (PR) positivity.[2]
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors
have been established as the standard of care in hormone
receptor–positive, human epidermal growth factor
receptor 2 (HER2)–negative advanced breast cancer not
at risk of imminent visceral compromise,[3] based on their
superior treatment effect in landmark registration trials
(i.e., the clinical trials that led the drugs to their approval
by the United States Food and Drug Administration
[FDA]).[4] [5] [6] [7] [8] [9] [10] [11] [12]
Palbociclib, ribociclib, and abemaciclib are the three
CDK4/6 inhibitors currently available in Hong Kong.[13] [14] [15]
Abemaciclib was not made available in the Hospital
Authority Drug Formulary until 11 July 2020.[16]
We hereby present our data in a real-life cohort of patients
from an institution in Hong Kong. We aimed to analyse
the safety and tolerability of palbociclib and ribociclib
in our centre. We sought to evaluate for any association between various clinicopathological and treatmentrelated
factors such as dose reduction or occurrences
of dose delay and treatment outcome, and whether the
treatment outcomes demonstrated in international trials
were reproducible in Asians.
METHODS
Data Collection
All patients who received palbociclib or ribociclib for
treating advanced breast cancer (including inoperable
stage III or stage IV, as per the eighth edition of the
American Joint Committee on Cancer staging manual)
during the period from July 2016 to February 2022
at Pamela Youde Nethersole Eastern Hospital were
included in the study. Medical records were reviewed
for data collection.
Study Objectives
The primary objective of this study was the safety and
tolerability of treatment, as measured by the frequencies
of adverse events (AEs) and need for dose reductions.
Toxicities were charted based on patients’ self-reported
symptoms and the regular review of laboratory results
before each cycle. AEs were graded according to the
Common Terminology Criteria for Adverse Events
version 5.0.
The secondary objective was the treatment outcome as
reflected by the progression-free survival (PFS), which
is defined as the time from treatment commencement
until the date of clinical or radiological progression of
measurable disease or death due to any cause. Disease
was assessed by physical examination at each visit and
regular imaging, including computed tomography or
positron emission tomography–computed tomography
scan that was usually performed at 4- to 6-month
intervals. Patients were followed up from date of
CDK4/6 inhibitor commencement till date of disease
progression or death.
Patients without evidence of disease progression at the
time of data cut-off (on 30 November 2022) or those who
defaulted follow-up were censored. Those who developed
disease progression or expired due to any cause during
treatment were defined as having had an event.
Statistical Analysis
Statistical analysis was conducted by SPSS (Windows
version 26.0; IBM Corp, Armonk [NY], United States).
Continuous variables were analysed by independent
sample t tests. Categorical variables were analysed
by Pearson’s Chi squared test or Fisher’s exact test.
The Kaplan–Meier method was used for estimation
of the PFS, with comparison made via the log-rank
test. The effects of multiple patient factors and of
clinicopathological and treatment-related factors on the
PFS were studied by the Cox proportional hazard model.
Factors deemed statistically significant (with a p value
< 0.05) on univariate analysis were further analysed by
multivariable analysis.
This manuscript was prepared in accordance with the
STROBE (Strengthening the Reporting of Observational
Studies in Epidemiology) guidelines.
RESULTS
Patient Demographics
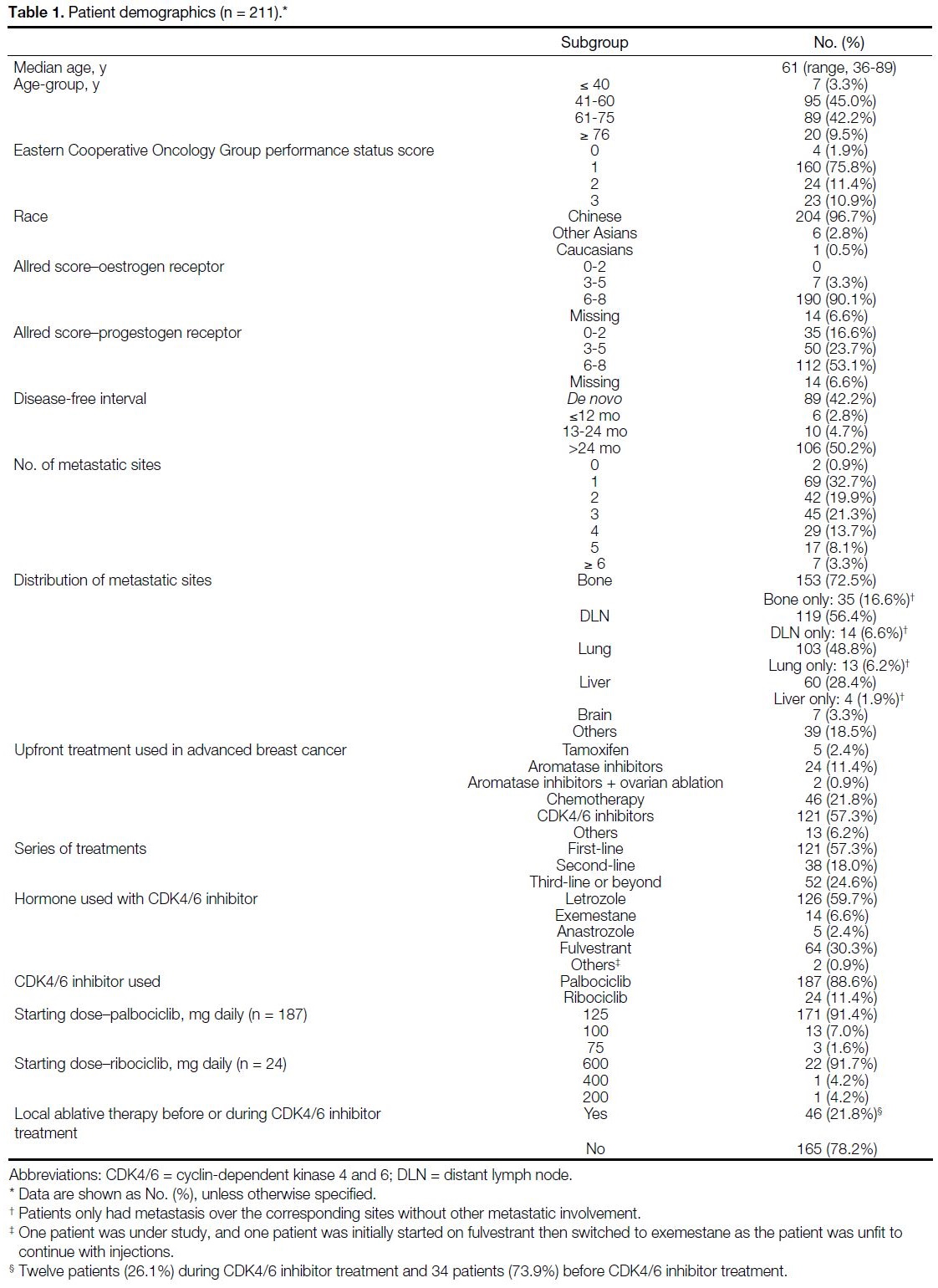
Patient demographics are detailed in Table 1. A total of
211 female patients with a median age of 61 years (range,
36-89) were included in the study, of which 96.7%
were Chinese, 2.8% were other Asians, and 0.5% were
Caucasians. A total of 77.7% had an Eastern Cooperative
Oncology Group performance status (ECOG PS) score
of 0-1 at the start of treatment, and 11.4% and 10.9%
were of ECOG PS score of 2 or 3, respectively. A total
of 88.6% received palbociclib and 11.4% received
ribociclib, with 57.3% receiving palbociclib or ribociclib
as first-line therapy and 18.0% and 24.6% receiving one of the drugs for second-line, third-line, or beyond.
The median duration of follow-up was 387 days (range, 5-2024).
Table 1. Patient demographics (n = 211).
Primary Outcome: Treatment Safety and
Tolerability
Occurrence of Adverse Events
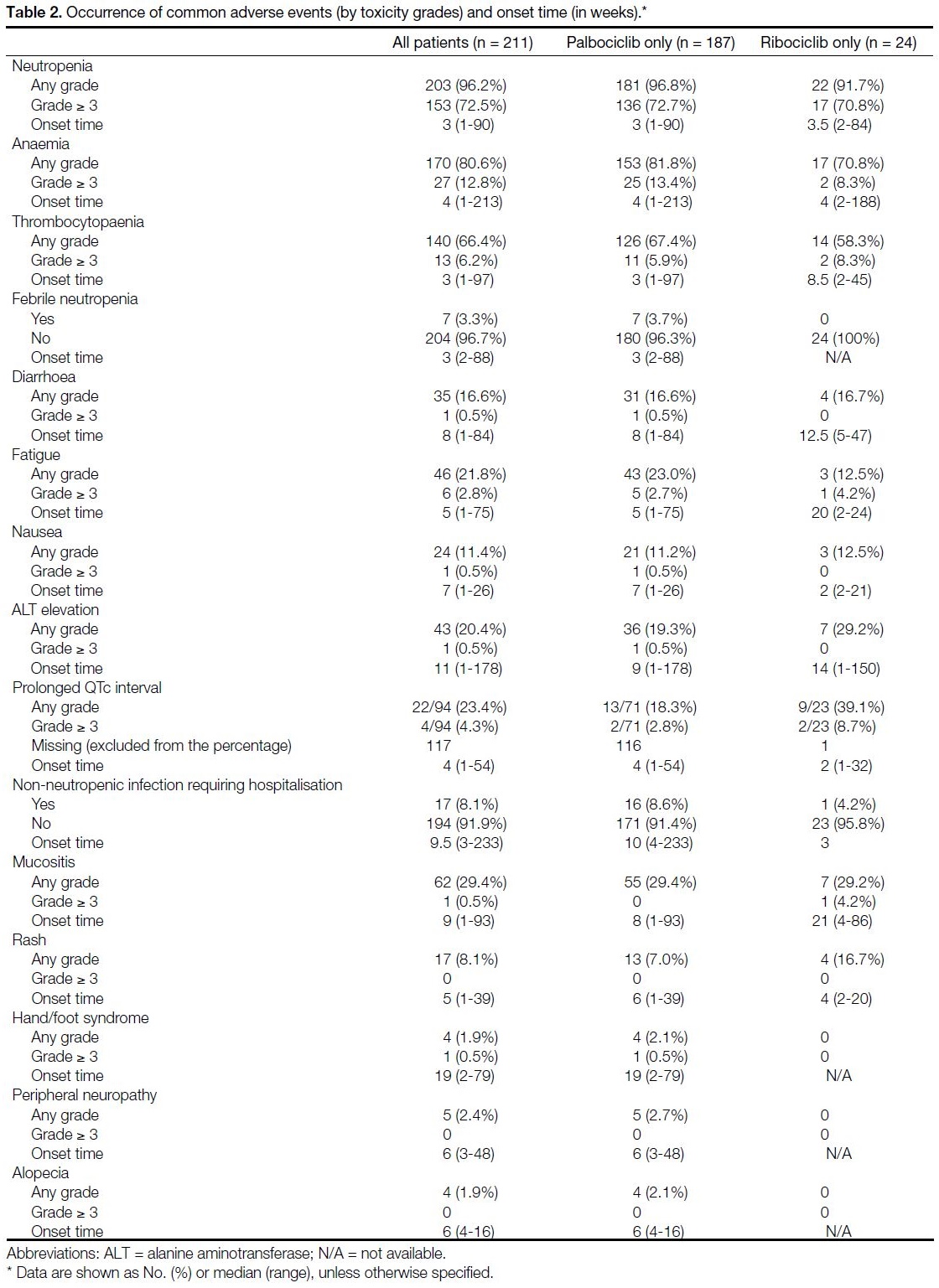
The occurrence of AEs (by toxicity grades) and
onset time (in weeks) are displayed in Table 2. The
most commonly observed AE of all grades was
neutropenia (96.2%), followed by anaemia (80.6%) and
thrombocytopaenia (66.4%). In all, 72.5% experienced
Grade ≥ 3 neutropenia, though the overall incidence
of febrile neutropenia was low (3.3%). Of note, one
patient (0.5%) experienced grade 5 hyperbilirubinaemia
and grade 4 thrombocytopaenia after 5.72 months of
ribociclib, dying of liver failure.
Table 2. Occurrence of common adverse events (by toxicity grades) and onset time (in weeks).
Occurrence of Dose Adjustments
In all, 171 out of 187 patients (91.4%) on palbociclib and 22 out of 24 patients (91.7%) on ribociclib were started
on the standard dose (Table 1). The remaining patients
were started on a reduced dose due to advanced age or
poor ECOG PS upon their physician’s discretion.
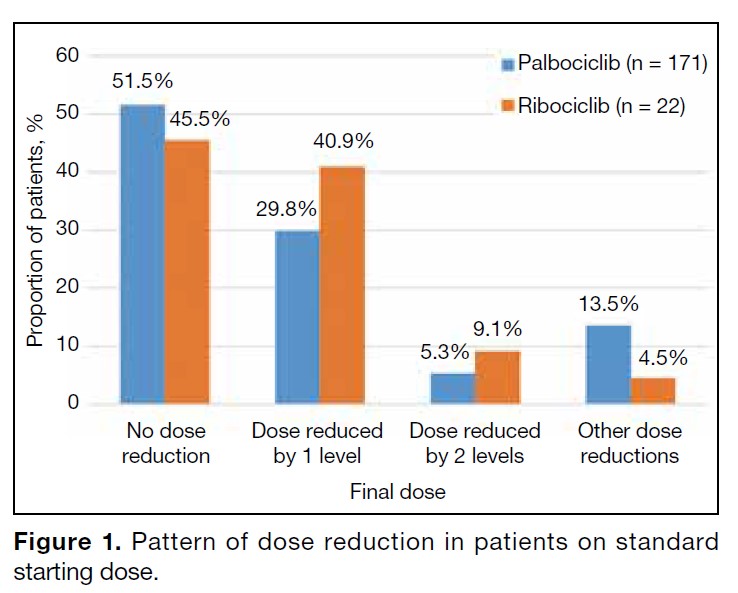
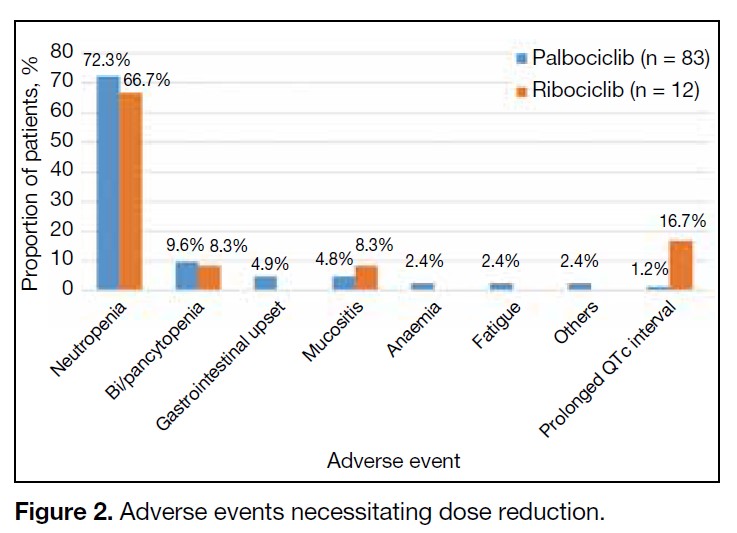
Among the patients on the standard starting dose, 48.5%
on palbociclib and 54.5% on ribociclib required dose
reduction, most commonly due to neutropenia. The
pattern and cause of dose reduction among patients on
standard starting doses are shown in Figures 1 and 2,
respectively. Dose reduction was largely adherent to
the recommended dosing levels of the FDA. Other dose
reductions included five weekly cycles or ‘2 weeks on 2
weeks off’ regimens.
Figure 1. Pattern of dose reduction in patients on standard starting dose.
Figure 2. Adverse events necessitating dose reduction.
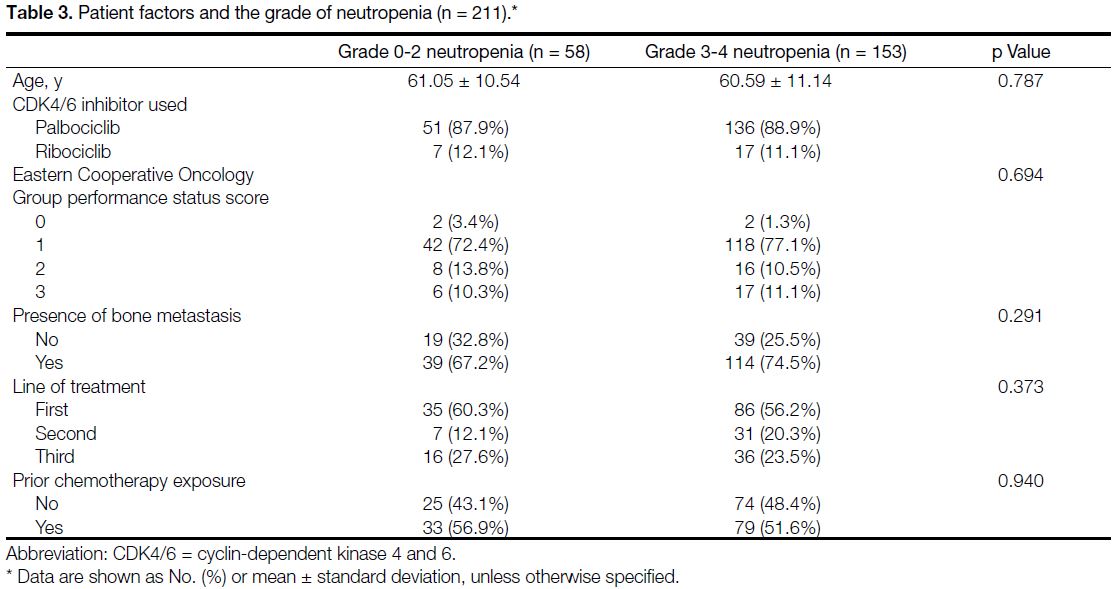
Patient Factors on the Presentation of Neutropenia
We analysed the association of multiple patient factors on the grade of neutropenia, including age, ECOG PS score,
presence of bone metastasis, first-line vs. later treatment,
and prior chemotherapy exposure. No statistically
significant predicting factors could be identified. The
results are detailed in Table 3.
Table 3. Patient factors and the grade of neutropenia (n = 211).
Secondary Outcome: Treatment Outcome
At the time of data cut-off, 59% (n = 125) patients
experienced an event of which 56% (n = 119) experienced
disease progression and 3% (n = 6) died due to causes
unrelated to oncological illness. A total of 40% (n = 84)
patients did not experience disease progression. A total
of 1% of patients (n = 2) were censored due to lack of
follow-up.
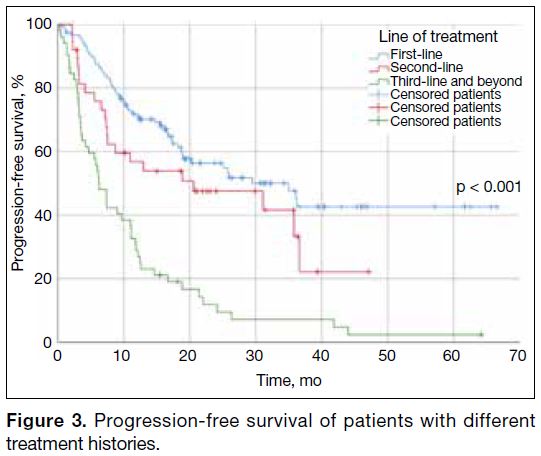
Patients who received palbociclib or ribociclib as first-line therapy enjoyed longer PFS than those at later
lines. Median PFS for first-, second-, and third-line and
beyond were 35 months, 20.6 months, and 6.2 months,
respectively (Figure 3).
Figure 3. Progression-free survival of patients with different treatment histories.
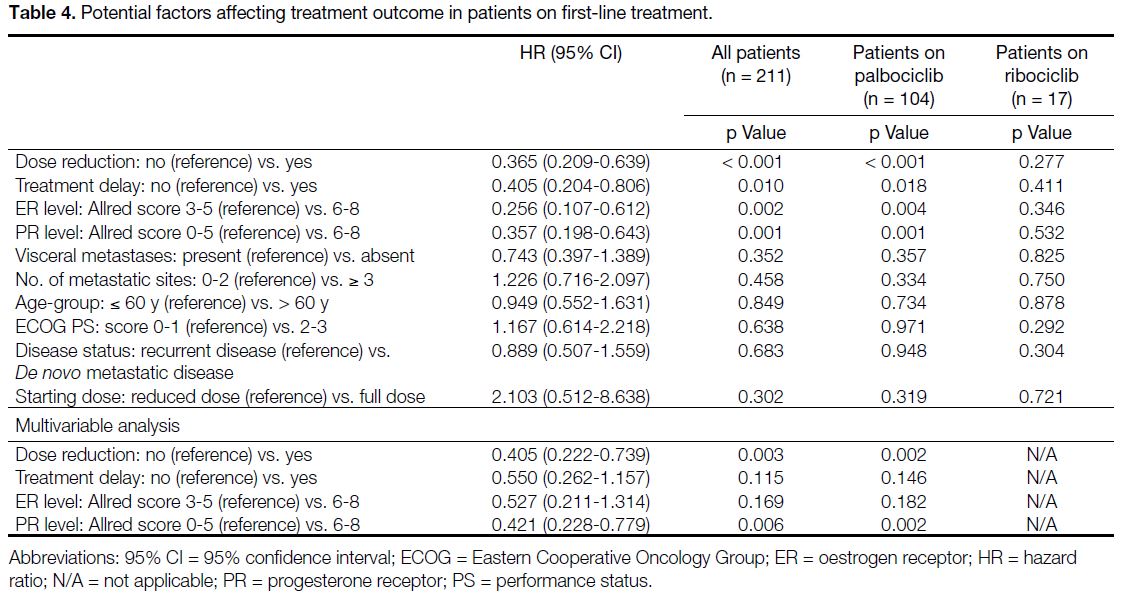
Potential Factors Affecting Treatment Outcome in Patients on First-Line Treatment
Dose reduction, treatment delay (defined by any delay
between cycles of ≥ 2 weeks), and high ER and PR
levels (with Allred score of 6-8) were each associated
with longer PFS, with p values of < 0.001, 0.010,
0.002, and 0.001, respectively. The absence of visceral
involvement was not statistically significant (p = 0.352).
There was no statistically significant difference in PFS
between younger and older age-groups (defined by cut-off
at 60 years old), nor between those with ECOG PS
scores of 0-1 and 2-3. Subsequent multivariable analysis
illustrated that dose reduction and strong PR levels
were predictors of longer PFS. ER levels and treatment
delay were significant factors in univariate analysis, but
such statistical significance was lost upon multivariable
analysis. Results are detailed in Table 4 and online supplementary Figure.
Table 4. Potential factors affecting treatment outcome in patients on first-line treatment.
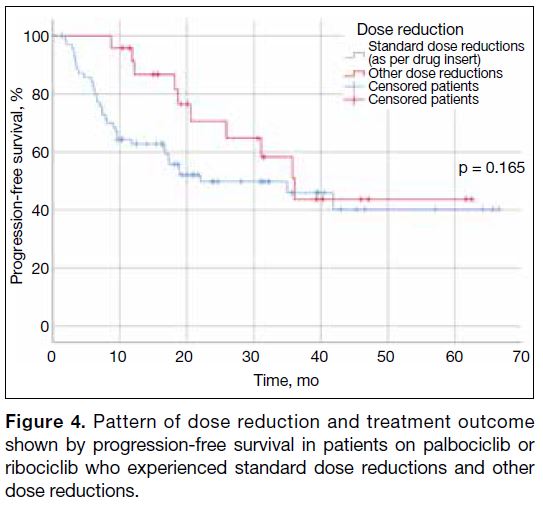
Pattern of Dose Reduction on the Treatment
Outcome
Amongst the patients started on standard dose regimens
who subsequently required dose reductions (n = 95), no statistically significant differences in PFS could be
observed between those who underwent dose reductions
adherent to the FDA drug insert, and those who received
dose reductions of other dosing regimens (Figure 4).
Figure 4. Pattern of dose reduction and treatment outcome shown by progression-free survival in patients on palbociclib or ribociclib who experienced standard dose reductions and other dose reductions.
DISCUSSION
The treatment landscape of advanced hormonere-sponsive,
HER2-negative breast cancer has transformed
dramatically since the emergence of CDK4/6 inhibitors.
Palbociclib demonstrated a median PFS of 24.8 and
11.2 months as first- or second-line treatment in
PALOMA-2[4] and 3[5] studies, respectively. Ribociclib
exhibited consistent PFS advantage in first- and
second-line treatment, and in premenopausal women in
MONALEESA-2,[6] [7] 3,[8] and 7[9] studies (median PFS = 20.5-25.3 months), with updated results revealing a
12.5-month overall survival benefit in first-line therapy.[10]
First-line abemaciclib also showed a superior PFS of 28.2 months compared to aromatase inhibitors alone in MONARCH-3 trial.[11] [12]
However, Asian patients are often underrepresented in
such clinical trials, with only 14.6% and 20% included
in PALOMA-2[4] and 3[5] studies, respectively, and 8.4%,
9.3%, and 30% included in MONALESSA-2,[6] [7] 3,[8] and 7[9] studies, respectively. There are various cohorts reporting
clinical outcomes of CDK4/6 inhibitors around Asia.[17] [18] [19] [20] [21] [22]
While the recently published PALOMA-4 trial recruited
patients from 52 centres across Asia, patients were all
of ECOG PS score of 0-1,[21] which was different from
the average patients we encounter in our daily clinical
practice.
Spanning the dates from July 2016 to February 2022, our
study population was predominantly on palbociclib with
nearly a quarter of patients not being exposed to CDK4/6
inhibitors unless on third-line therapy or beyond. Such
practice was largely influenced by drug availability in
our locality. Palbociclib, ribociclib, and abemaciclib
were registered in Hong Kong on 2 December 2016,[13] 26
January 2018,[14] and 18 December 2019,[15] respectively.
Before their corresponding inclusion into the Hospital
Authority Drug Formulary on 14 July 2018,[23] 13 October
2018,[24] and 11 July 2020,[16] patients had to receive
such treatment on a named patient basis. The monthly
treatment cost of HKD$18,000 to $23,100 precluded its
initial accessibility within our public hospital setting,
with more widespread use of palbociclib and ribociclib
observed since their coverage under the Community
Care Fund on 12 January 2019[25] and 13 July 2019,[26]
respectively. Abemaciclib was not covered by the Fund
till 9 January 2021,[27] and none of our patients in this
study received it.
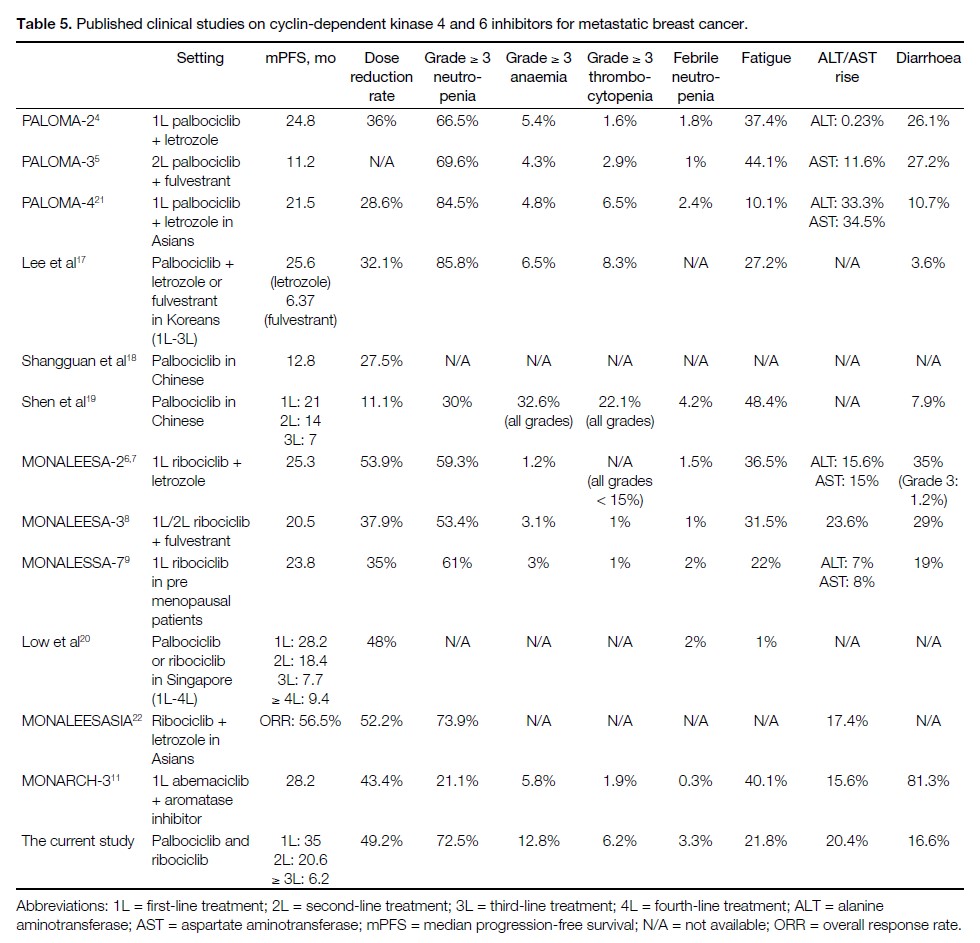
Table 5 displays the median PFS, dose reduction rate,
and frequency of toxicities reported in landmark clinical
trials and other regional cohorts, compared with our
experience. The longer median PFS in first- and secondline
treatment in our cohort as compared to those reported
in landmark clinical trials could be due to the less unified
timing of response assessment in the real-world setting
and the relatively short median follow-up time of 12.6
months. While acknowledging that direct comparisons
of the PFS with landmark clinical trials are hindered by
our heterogeneous patient group, the overall pattern of
treatment outcome remains comparable to international
standards.
Table 5. Published clinical studies on cyclin-dependent kinase 4 and 6 inhibitors for metastatic breast cancer./div>
Our dose reduction rates were higher than those of the PALOMA-2 study[4] but similar to those reported in the MONALEESA-2 study[6] [7] and other regional Asian cohorts.
Higher rates of grade ≥ 3 haematological toxicities
were seen in our cohort than in PALOMA-2 or 3 and
MONALEESA-2, 3, or 7 studies, but they were similar
to those in PALOMA-4 study and other regional Asian
cohorts. This echoes the reports of a higher incidence
of grade ≥ 3 neutropenia in Asians, which can be up to
92%.[28] Febrile neutropenia remained low.
Consistent with other international cohorts,[18] [19] [28] [29] [30] [31] dose reduction did not compromise the treatment efficacy.
In a detailed safety analysis of the PALOMA-2 study,
Diéras et al[29] conducted a landmark analysis of dose
reduction on treatment efficacy at 3, 6, and 9 months in
the palbociclib arm. It showed similar PFS in patients
who experienced dose reduction and those who did not.[29]
Similarly, a pooled safety analysis of MONALEESA-2,
3 and 7 studies showed similar median PFS of 24.8 to
29.6 months across patients on various dose intensities
that ranged from ≤ 71% to 100%, which reaffirmed that
the PFS, overall response rate, and clinical benefit rate
were maintained regardless of dose modifications.[30] One
suggestion made by the authors was that variations in drug
metabolism and pharmacodynamic effects were present
such that patients who experienced more treatment-related
AEs, hence requiring dose modifications, were
also subjected to enhanced drug exposure leading to
an enhanced therapeutic effect.[30] Further studies are
warranted to explore the reasons behind this.
Interestingly, we reproduced the same observations
noted in a multicentre study from the United Kingdom,[31]
in which dose reduction and delays were associated with
longer PFS. Owing to the retrospective nature of our
study consisting of a relatively small and heterogeneous
population, we do not aim to conclude superiority of such
dose alterations over the standard dose. Nonetheless, this
could be a reassurance that de-escalation of palbociclib
or ribociclib dose can be considered in patients when
deemed clinically necessary without compromising the
treatment outcome.
High PR levels were identified as a statistically significant
predictor of longer PFS. High ER levels trended towards
statistically significant longer PFS only on univariate
but not multivariate analysis. This could be limited by
the small number of low–ER level patients (3.3%, n = 7) in our cohort, precluding analysis. Nevertheless, this
mirrored with the observation of PR levels—the other
surrogate marker for endocrine responsiveness—and
resonates with the exploratory analysis results of the
PADA-1 trial presented at the European Society for
Medical Oncology Breast Cancer Congress 2022.[32]
ER and PR immunohistochemistry scores were shown
to have significant impact on PFS achieved with first-line
palbociclib, with each 10% gain in ER level being
associated with a 10% reduced risk of PFS events (hazard
ratio = 0.90; p = 0.002), and each 10% gain in PR level being associated with a 8% risk of PFS events (hazard
ratio = 0.92; p < 0.001).[32] This could be a potential area
for future studies, aiming to elucidate whether patients
with higher ER and PR levels could benefit from a
tailor-made reduced dose while still achieving similar
therapeutic effect.
Limitations
Limited by its retrospective nature, our clinical study
was inevitably influenced by environmental factors such
as the varying availability of the drugs due to time and cost constraints, deviation from standard dose reduction
protocol, and underreporting of non-haematological
toxicities leading to minor deviations from international
cohorts. Nonetheless, the clinical outcomes and safety
profile of palbociclib and ribociclib in our centre largely
mirrored that seen in drug registration clinical trials and
other regional Asian cohorts.
CONCLUSION
To our knowledge, this is the largest cohort of its kind
reported locally in Hong Kong. The treatment outcomes
and safety profiles of palbociclib and ribociclib
demonstrated in our institution, with a predominantly
Asian population with ECOG PS scores ranging from 0
to 3, were comparable to those quoted in international
clinical trials and other regional Asian cohorts. Dose
reduction could be considered when deemed clinically
necessary, in hopes of maximising patients’ tolerance
to treatment and maintaining patients’ quality of life,
either upon treatment commencement in perhaps older
and frailer patients, or in face of AEs, as it did not
compromise the treatment efficacy.
REFERENCES
1. Hong Kong Cancer Registry, Hospital Authority, Hong Kong SAR
Government. Leader cancer sites in Hong Kong in 2019. Available
from: https://www3.ha.org.hk/cancereg/pdf/top10/rank_2019.pdf. Accessed 11 Dec 2022.
2. Hong Kong Breast Cancer Foundation. Hong Kong Breast Cancer Registry Report No. 12. 2020. Available from: http://www.hkbcf.org/en/our_research/main/519/upload/category/519/self/5f7bdc6663cf9.pdf. Accessed 11 Dec 2022.
3. Gennari A, André F, Barrios CH, Cortés J, de Azambuja E,
DeMichele A, et al. ESMO Clinical Practice Guideline for the
diagnosis, staging and treatment of patients with metastatic breast
cancer. Ann Oncol. 2021;32:1475-95. Crossref
4. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925-36. Crossref
5. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N,
et al. Overall survival with palbociclib and fulvestrant in advanced
breast cancer. N Engl J Med. 2018;379:1926-36. Crossref
6. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738-48. Crossref
7. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS,
Paluch-Shimon S, et al. Updated results from MONALEESA-2, a
phase III trial of first-line ribociclib plus letrozole versus placebo
plus letrozole in hormone receptor–positive, HER2-negative
advanced breast cancer. Ann Oncol. 2018;29:1541-7. Crossref
8. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im
SA, et al. Phase III randomized study of ribociclib and fulvestrant
in hormone receptor–positive, human epidermal growth factor
receptor 2–negative advanced breast cancer: MONALEESA-3. J
Clin Oncol. 2018;36:2465-72. Crossref
9. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor–positive, advanced breast cancer
(MONALEESA-7): a randomised phase 3 trial. Lancet Oncol.
2018;19:904-15. Crossref
10. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS,
Hart L, et al. Overall survival with ribociclib plus letrozole in
advanced breast cancer. N Engl J Med. 2022;386:942-50. Crossref
11. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638-46. Crossref
12. Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. Crossref
13. Drug Office, Department of Health, Hong Kong SAR Government.
Drug database—Ibrance capsules 125 mg. Available from: https://www.drugoffice.gov.hk/eps/drug/productDetail2/en/consumer/138597. Accessed 9 Dec 2022. Crossref
14. Drug Office, Department of Health, Hong Kong SAR Government.
Drug database—Kisqali tablets 200 mg. Available from:
https://www.drugoffice.gov.hk/eps/drug/productDetail2/en/consumer/125912. Accessed 9 Dec 2022. Crossref
15. Drug Office, Department of Health, Hong Kong SAR Government.
Drug database—Verzenio tablets 150 mg. Available from:
https://www.drugoffice.gov.hk/eps/drug/productDetail2/en/consumer/124855. Accessed 9 Dec 2022. Crossref
16. Hospital Authority, Hong Kong SAR Government. Update of Hospital Authority Drug Formulary - version 16.1. 2020 July 11.
17. Lee J, Park HS, Won HS, Yang JH, Lee HY, Woo IS, et al. Real-world clinical data of palbociclib in Asian metastatic breast cancer patients: experiences from eight institutions. Cancer Res Treat. 2021;53:409-23. Crossref
18. Shangguan CF, Jiang M, Yang C, Lou GY, Li YT, Qu Q. Clinical
efficacy of palbociclib-based therapy in women with HR+/HER2-metastatic breast cancer in the real-world setting for Chinese
women: a comparison with the IRIS study. Eur Rev Med Pharmacol
Sci. 2021;25:6138-48. Crossref
19. Shen L, Zhou J, Chen Y, Ding J, Wei H, Liu J, et al. Treatment
patterns, effectiveness, and patient-reported outcomes of
palbociclib therapy in Chinese patients with advanced breast
cancer: a multicenter ambispective real-world study. Cancer Med.
2022;11:4157-68. Crossref
20. Low JL, Lim E, Bharwani L, Wong A, Wong K, Ow S, et al. Real-world outcomes from use of CDK4/6 inhibitors in the management of advanced/metastatic breast cancer in Asia. Ther Adv Med Oncol. 2022;14:17588359221139678. Crossref
21. Xu B, Hu X, Li W, Sun T, Shen K, Wang S, et al. Palbociclib plus
letrozole versus placebo plus letrozole in Asian postmenopausal
women with oestrogen receptor–positive/human epidermal growth
factor receptor 2–negative advanced breast cancer: primary results
from PALOMA-4. Eur J Cancer. 2022;175:236-45. Crossref
22. Yap YS, Chiu J, Ito Y, Ishikawa T, Aruga T, Kim SJ, et al. Ribociclib, a CDK 4/6 inhibitor, plus endocrine therapy in Asian women with advanced breast cancer. Cancer Sci. 2020;111:3313-26. Crossref
23. Hospital Authority, Hong Kong SAR Government. Update of Hospital Authority Drug Formulary - version 14.1. 2018 July 14.
24. Hospital Authority, Hong Kong SAR Government. Update of Hospital Authority Drug Formulary - version 14.2. 2018 October 13.
25. Hospital Authority, Hong Kong SAR Government. Update of Hospital Authority Drug Formulary - version 14.3. 2019 January 12.
26. Hospital Authority, Hong Kong SAR Government. Items supported
by the CCF Medical Assistance Programmes. Available from:
https://www.ha.org.hk/visitor/ha_visitor_index.asp?content_id=208076. Accessed 15 Feb 2024.
27. Hospital Authority, Hong Kong SAR Government. Update of Hospital Authority Drug Formulary - version 16.3. 2021 January 9.
28. Verma S, Bartlett CH, Schnell P, DeMichele AM, Loi S, Ro J, et al.
Palbociclib in combination with fulvestrant in women with hormone
receptor–positive/HER2-negative advanced metastatic breast
cancer: detailed safety analysis from a multicenter, randomised,
placebo-controlled, phase III study (PALOMA-3). Oncologist.
2016;21:1165-75. Crossref
29. Diéras V, Harbeck N, Joy AA, Gelmon K, Ettl J, Verma S, et al.
Palbociclib with letrozole in postmenopausal women with ER+/HER2- advanced breast cancer: haematologic safety analysis of
the randomized PALOMA-2 trial. Oncologist. 2019;24:1514-25. Crossref
30. Burris HA, Chan A, Bardia A, Thaddeus Beck J, Sohn J, Neven P,
et al. Safety and impact of dose reductions on efficacy in the
randomised MONALEESA-2, -3 and -7 trials in hormone receptor–positive, HER2-negative advanced breast cancer. Br J Cancer.
2021;125:679-86. Crossref
31. El Badri S, Tahir B, Balachandran K, Bezecny P, Britton F,
Davies M, et al. Palbociclib in combination with aromatase
inhibitors in patients ≥75 years with oestrogen receptor–positive,
human epidermal growth factor receptor 2–negative advanced
breast cancer: a real-world multicentre UK study. Breast.
2021;60:199-205. Crossref
32. De La Motte Rouge T, Frenel JS, Hardy-Bessard AC, Bachelot T,
Pistilli B, Delaloge S, et al. 167MO — Association between ER, PR
and HER2 levels and outcome under palbociclib (Pal) + aromatase
inhibitors (AIs) as first-line therapy for ER+/HER2- metastatic
breast cancer (MBC): an exploratory analysis of the PADA-1 trial.
Ann Oncol. 2022;33 Suppl 3:S200. Crossref