Endovascular Management of Iatrogenic Neck Vascular Injury After Central Venous Catheterisation
PICTORIAL ESSAY
Hong Kong J Radiol 2024 Mar;27(1):e65-74 | Epub 12 March 2024
Endovascular Management of Iatrogenic Neck Vascular Injury After Central Venous Catheterisation
SC Wong, SY Lam, SKH Wong, HF Chan, LF Cheng
Department of Radiology, Princess Margaret Hospital, Hong Kong SAR, China
Correspondence: Dr SC Wong, Department of Radiology, Princess Margaret Hospital, Hong Kong SAR, China. Email: wsc696@ha.org.hk
Submitted: 7 October 2022; Accepted: 4 January 2023.
Contributors: All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript
for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Kowloon West Cluster Research Ethics Committee of Hospital Authority, Hong Kong [Ref No.: KW/EX-22-026(170-02)]. The patients were treated in accordance with the tenets of the Declaration of Helsinki. Informed consent from
patient was waived by the Committee due to anonymisation and secure storage of all patient data.
Acknowledgement: The authors thank Dr WK Ng, Dr TW Yan and Dr KM Chan, vascular surgeons from the Department of Surgery of Princess Margaret Hospital, for their contribution to the endovascular treatment and surgical dissection in the cases illustrated in the study.
INTRODUCTION
Central venous line placement is a common procedure
in both elective and emergency settings across different
medical and surgical specialties. The jugular and
subclavian approaches are common methods of central
venous catheterisation in the neck, which can be
performed by traditional methods based on anatomical
landmarks or under ultrasound guidance.[1]
Though rare, iatrogenic neck arterial injury can occur
during attempts at central venous catheterisation, leading
to serious complications such as arterial perforation with
active bleeding, pseudoaneurysm, arteriovenous fistula,
arterial dissection, or vascular occlusion resulting in
neurological or ischaemic sequelae. Risk factors include
obesity, short neck, and emergency catheterisation.[2] [3]
Surgical approaches to repair injured arteries may involve
extensive dissection or open-cardiothoracic surgical
repair and vascular grafting, and often require general anaesthesia. These may entail prolonged recovery and
may not be tolerated by critically ill or elderly patients.
Endovascular management of central venous line
complications and retrieval of retained indwelling
catheters or their components can be a promising first-line
treatment option in view of its minimally invasive
nature, high success rate with reduced morbidity, and
enhanced recovery compared with open surgery. It may
be performed under local anaesthesia.
Treatment plans for arterial complications should
be individualised based on the type of complication,
relationship to adjacent vital vessels, angiographic
factors, and patients’ underlying health conditions.
The pros and cons of different endovascular treatment
options need to be examined.
With increasing use of long-term indwelling venous catheters, it is vital that clinicians be aware of the
associated risks of retained catheter component(s) within
veins or failed catheter removal due to an adherent fibrin
sheath. Forceful removal may further jeopardise blood
vessels or result in device fragmentation or embolisation.
This article reviews cases of successful treatment of the iatrogenic complications of neck arterial catheterisation
during central venous catheterisation as well as of
unintentionally retained catheters or their fragments from
neck veins, with illustration of different endovascular
treatment techniques.
ARTERIAL INJURY
The subclavian, brachiocephalic, and carotid arteries are in close proximity to the internal jugular and subclavian
veins, placing them at risk of iatrogenic injury during
venous catheterisation. Reflux of pulsatile or fresh
blood within the catheter, neck haematoma, abnormal
catheter course, or acute neurological deficit should raise
suspicion for arterial injury. The risk of complications
increases with the calibre of the device that punctured
the artery.[4] [5]
In the setting of iatrogenic carotid artery puncture by
small-calibre vascular access needles (e.g., ≤ 20-G), the
risk of major complications is relatively low, with needle
removal and external compression being a feasible
management option with a low complication rate.[5]
However, in the case of subclavian or brachiocephalic
artery injury, haemostasis by compression alone may
be ineffective due to lack of an underlying bone to
facilitate compression in the supraclavicular region, and
is associated with a higher complication rate.[3]
Ideally, operators should confirm the venous location of
the vascular access needle prior to insertion of large-bore
vascular dilator, sheath or catheter, because of the higher
complication rates if these instruments are unintentionally
introduced into the arterial system.[4] If iatrogenic large-bore catheter injury occurs (such as insertion of a catheter > 7 Fr or a vessel dilator[4] [5]), operators should
refrain from immediate withdrawal of the malpositioned
catheter from major arteries, as the catheter can still
tamponade the vessel, limiting bleeding and providing
an endovascular access route for closure of the arterial
perforation. Intravascular balloon tamponade against the
arterial puncture site for temporary haemostasis can also
be helpful while contemplating definitive treatment. A
misplaced dilator or large-bore catheter should be left in place as recommended by the Practice Guidelines
for Central Venous Access 2020 by the American
Society of Anesthesiologists[4] prior to removal. Urgent
resuscitation, multidisciplinary consultation with
interventional radiologists, vascular or cardiothoracic
surgeons should be sought to devise an individualised
treatment plan. If the site of arterial injury is clearly
visible and surgically accessible, treatment options may
include direct surgical arterial repair[5] or endovascular
treatment. However, when the site of arterial injury is
not well defined or easily accessible surgically, prompt
imaging evaluation[5] such as computed tomographic
angiography is pivotal in treatment planning and
assessing complications.
Different endovascular treatment options can be used
sequentially or in combination to achieve haemostasis,
which comprise endovascular treatment such as
stenting, embolisation, or coiling of a pseudoaneurysm
using a percutaneous approach. Embolisation or
coiling of a pseudoaneurysm may be performed if the
pseudoaneurysm sac can be selectively cannulated,
which may be technically challenging in the neck region
especially if the injured feeding vessel is tortuous.
Endovascular Stenting
Covered stent graft deployment along the injured segment
is helpful in preserving perfusion, with exclusion of
the pseudoaneurysm from circulation (Figure 1). This
is imperative if the injured artery must be preserved to
supply organs or extremities.
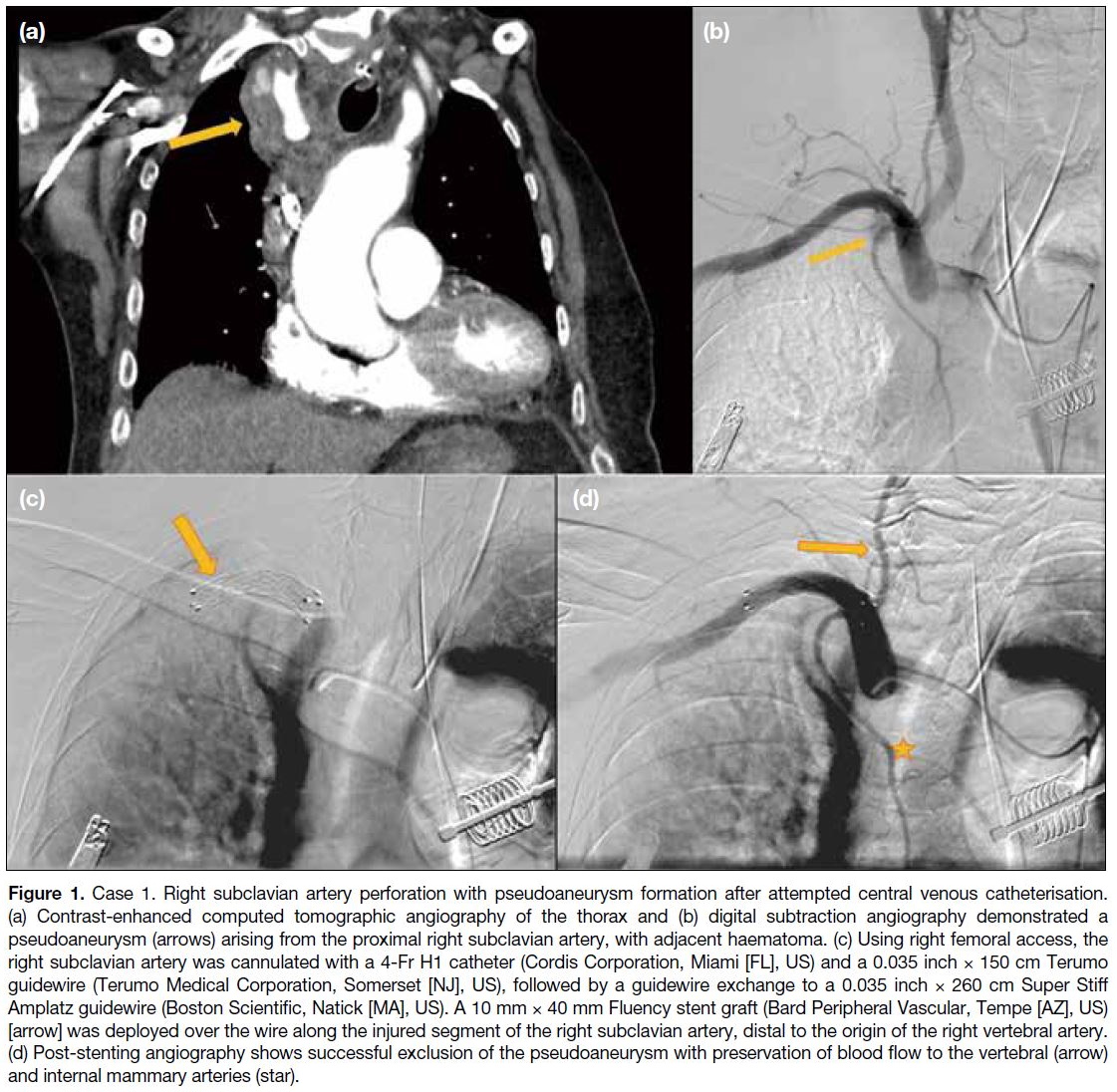
Figure 1. Case 1. Right subclavian artery perforation with pseudoaneurysm formation after attempted central venous catheterisation.
(a) Contrast-enhanced computed tomographic angiography of the thorax and (b) digital subtraction angiography demonstrated a
pseudoaneurysm (arrows) arising from the proximal right subclavian artery, with adjacent haematoma. (c) Using right femoral access, the
right subclavian artery was cannulated with a 4-Fr H1 catheter (Cordis Corporation, Miami [FL], US) and a 0.035 inch × 150 cm Terumo
guidewire (Terumo Medical Corporation, Somerset [NJ], US), followed by a guidewire exchange to a 0.035 inch × 260 cm Super Stiff
Amplatz guidewire (Boston Scientific, Natick [MA], US). A 10 mm × 40 mm Fluency stent graft (Bard Peripheral Vascular, Tempe [AZ], US)
[arrow] was deployed over the wire along the injured segment of the right subclavian artery, distal to the origin of the right vertebral artery.
(d) Post-stenting angiography shows successful exclusion of the pseudoaneurysm with preservation of blood flow to the vertebral (arrow)
and internal mammary arteries (star).
In the literature, both balloons and self-expanding
stents have been used in management of arterial injury.[6]
Among self-expanding stents, Fluency (Bard Peripheral
Vascular Inc, Tempe [AZ], US), Viabahn (WL Gore &
Associates Inc, Newark [DE], US), Wallgraft (Boston
Scientific, Natick [MA], US), and Cragg covered stents
(Boston Scientific, Natick [MA], US) have all been
used.[6] The covered stents available in the Hong Kong
market include the Fluency self-expanding stent, the
BeGraft peripheral balloon-mounted stent graft system
(Bentley InnoMed, Hechingen, Germany), the iCover
balloon-expandable stent (iVascular, Barcelona, Spain),
and the recently available Advanta V12 balloon-expandable
covered stent (Advanta, Getinge, Sweden).
Choice of stent graft should be based on device
availability, the operator’s experience, arterial diameter,
distance of other vital branches from the subclavian artery (especially the vertebral artery), and the availability of
adequate landing zones. An ideal covered stent should
have flexibility and conformability to the vessel, allowing
for adaptation to vascular tortuosity.
Caution should be exercised if stent deployment
potentially involves the vertebral artery origin, due to
the risk of impairing posterior circulation, resulting in
ischaemic stroke, especially if patients lack contralateral
dominant vertebral artery supply.
To the best of authors’ knowledge and experience, while
there is no universal consensus or guideline regarding
the use of antiplatelet drugs or anticoagulants after
emergency stenting, the clinical decision regarding
choice and timing of starting antiplatelet drugs and
anticoagulants after stenting should be based on balancing
the risks of rebleeding versus stent thrombosis, as well
as contraindications for antiplatelet or anticoagulant use
from patient co-morbidities (such as recent intracranial
haemorrhage).
In the long term, the decision for lifelong antiplatelet
treatment (e.g., lifelong aspirin 80 mg per oral daily)
would be made on a case-by-case basis, balancing the
risk of stent thrombosis versus the risk of long-term
antiplatelet use.
Vascular Closure Devices
Suture-mediated vascular closure devices have emerged
as an alternative treatment option and are particularly
favoured in frail patients in averting major open
surgical repair. Originally approved for percutaneous closure of femoral artery punctures, which are more
superficial, suture-mediated closure devices such as
Perclose ProGlide have been used off-label with reports
of successful closure of the subclavian or innominate
artery.[7] [8] The minimal amount of intraluminal material
used mitigates the risk of thromboembolism or device
dislodgement. To facilitate deployment of the closure
device as close to the vessel perforation site as possible
in a relatively deep arterial perforation site along the
subclavian or innominate artery, regional neck dissection
by vascular surgery to gain adequate vessel exposure
may be helpful (Figure 2).
Figure 2. Case 2. A malpositioned triple lumen central venous catheter perforates into the right subclavian artery. Chest X-ray (a)
demonstrates the abnormal course of the catheter with subclavian artery catheterisation and the tip (arrow) angulated towards the right
axilla. Digital subtraction angiography (b) shows the course of catheter within the right subclavian artery (arrow), with the site of arterial entry
distal to the origin of the vertebral artery and the tip in the right axillary artery. The interventional radiology team planned for removal of the
catheter with arterial repair by a vascular closure device. (c) A safety guidewire (260-cm Amplatz Super Stiff guidewire; Boston Scientific,
Natick [MA], US) was inserted via a right femoral artery sheath, coursing to the right brachial artery (star). Right lower neck dissection was
performed by the vascular surgeons to facilitate access to the subclavian artery for deployment of the vascular closure device and repair of
the internal jugular vein. The malpositioned catheter in the right subclavian artery was removed over a 0.035-inch Terumo guidewire (Terumo
Medical Corporation, Somerset [NJ], US). The right subclavian artery was repaired by Perclose ProGlide (Abbott Vascular, Redwood City
[CA], US) [arrow] deployed just adjacent to the subclavian artery at neck dissection. A subsequent right subclavian angiogram (d) reveals
a patent subclavian artery without contrast extravasation. The safety guidewire was then removed with neck wound closure by surgery.
Complications of suture-mediated closure devices
include complete occlusion of the artery[9] and failed
haemostasis. Therefore, placement of a safety guidewire
into the injured artery prior to deploying the vascular
closure device (Figure 2c) would facilitate rapid
deployment of a balloon for temporary occlusion, or
stenting as secondary haemostatic measures in case of
failure of vascular closure device.
Pseudoaneurysm Treatment with
Percutaneous Thrombin Injection
Percutaneous thrombin injection for pseudoaneurysms
is a safe and effective treatment with a success rate
of > 90%,[10] [11] which is useful in the supraclavicular
or retroclavicular region and is accessible with
percutaneous needle puncture or when the injured
artery is not accessible by endovascular catheterisation
(Figures 3 and 4). Slow injection under real-time imaging
guidance, with the needle tip directed away from the
pseudoaneurysm sac, help minimise the risk of non-target
embolisation. The advantages of this procedure
include simplicity, speed and less discomfort compared
with ultrasound-guided compression, as well as a low
complication rate (< 1.3%).[11]
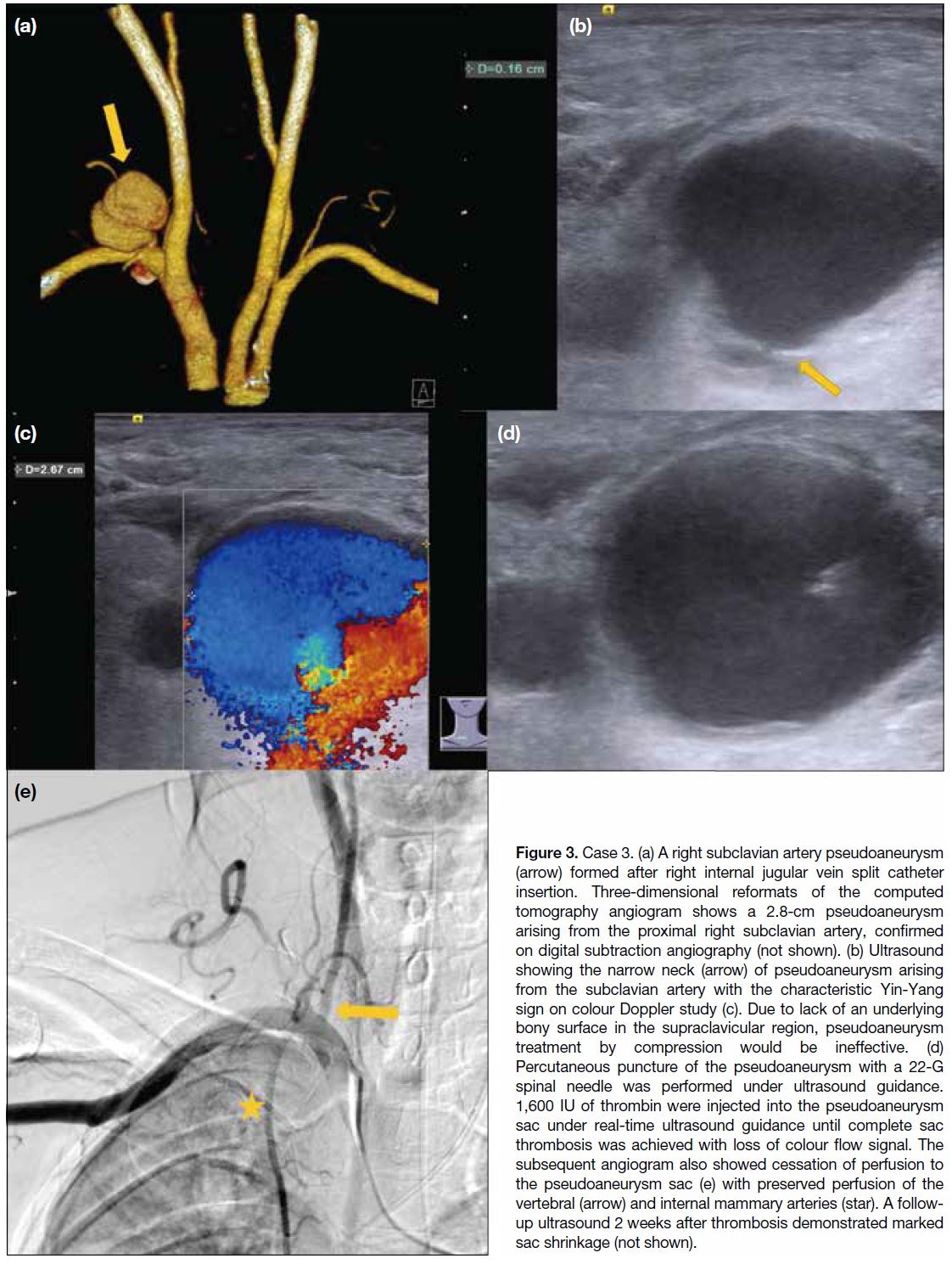
Figure 3. Case 3. (a) A right subclavian artery pseudoaneurysm
(arrow) formed after right internal jugular vein split catheter
insertion. Three-dimensional reformats of the computed
tomography angiogram shows a 2.8-cm pseudoaneurysm
arising from the proximal right subclavian artery, confirmed
on digital subtraction angiography (not shown). (b) Ultrasound
showing the narrow neck (arrow) of pseudoaneurysm arising
from the subclavian artery with the characteristic Yin-Yang
sign on colour Doppler study (c). Due to lack of an underlying
bony surface in the supraclavicular region, pseudoaneurysm
treatment by compression would be ineffective. (d)
Percutaneous puncture of the pseudoaneurysm with a 22-G
spinal needle was performed under ultrasound guidance.
1,600 IU of thrombin were injected into the pseudoaneurysm
sac under real-time ultrasound guidance until complete sac
thrombosis was achieved with loss of colour flow signal. The
subsequent angiogram also showed cessation of perfusion to
the pseudoaneurysm sac (e) with preserved perfusion of the
vertebral (arrow) and internal mammary arteries (star). A follow-up
ultrasound 2 weeks after thrombosis demonstrated marked
sac shrinkage (not shown).
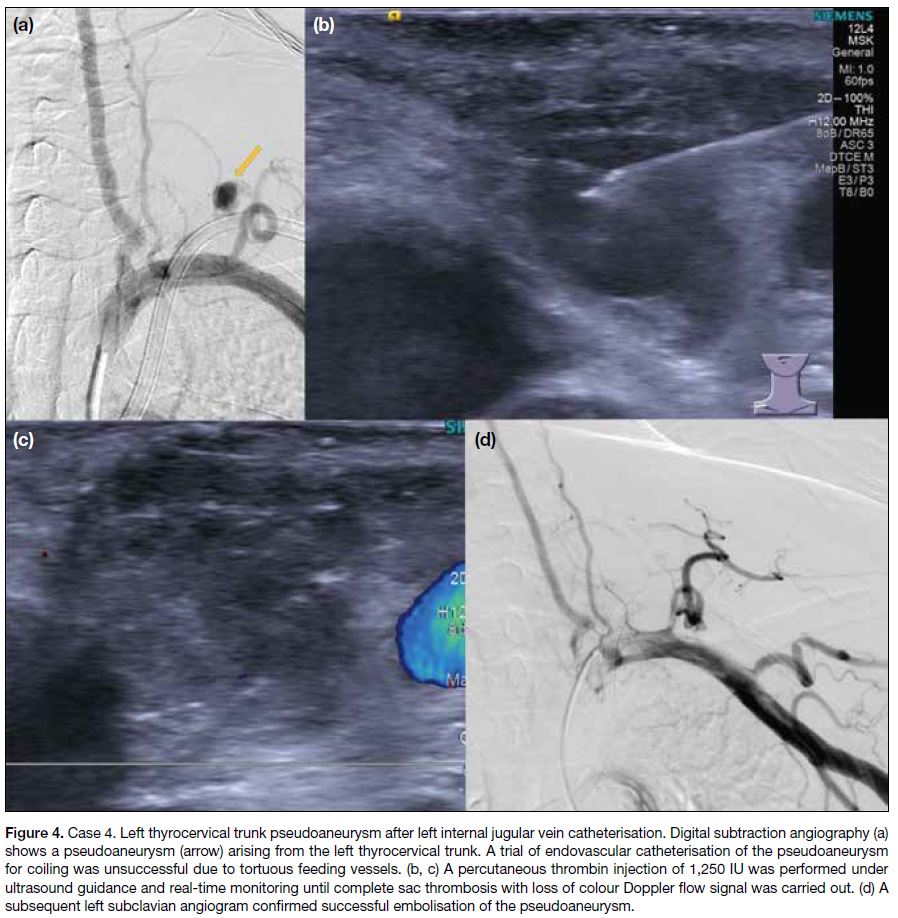
Figure 4. Case 4. Left thyrocervical trunk pseudoaneurysm after left internal jugular vein catheterisation. Digital subtraction angiography (a)
shows a pseudoaneurysm (arrow) arising from the left thyrocervical trunk. A trial of endovascular catheterisation of the pseudoaneurysm
for coiling was unsuccessful due to tortuous feeding vessels. (b, c) A percutaneous thrombin injection of 1,250 IU was performed under
ultrasound guidance and real-time monitoring until complete sac thrombosis with loss of colour Doppler flow signal was carried out. (d) A
subsequent left subclavian angiogram confirmed successful embolisation of the pseudoaneurysm.
A small risk of iatrogenic thrombin embolisation into
the parent artery resulting in arterial occlusion[11] exists,
which may be minimised with injection distant from
the pseudoaneurysm neck. Other risks include allergic
rection and infection such as skin cellulitis or abscess
formation.
RETRIEVAL OF BROKEN CATHETER FRAGMENTS
Mechanical failure of a long-term central venous catheter may result in catheter fragmentation, dislodgement, or
embolisation. Imaging with plain radiographs such as chest X-ray is readily available for prompt screening of
catheter integrity in clinical setting.
Computed tomography enables accurate localisation
of dislodged catheter fragments, assessment of the
relationship to adjacent vasculature, and detection of
complications. Imaging can facilitate assessment of
crucial factors in treatment planning, including the size,
orientation and location of dislodged catheter fragments,
the calibre of venous access routes, and the intended
extraction route and vascular access site, which can
guide the choice of type and size of retrieval device with
reference to available institutional resources.
Establishment of venous access through a large calibre
superficial vein such as the internal jugular or common
femoral vein is beneficial in enabling convenient
equipment deployment. Haemostasis in these superficial
venous access sites can be more easily achieved with
compression.[12] The relatively straightforward course
from the right internal jugular vein or femoral veins to
the vena cava with reduced angulation compared with
the left side facilitates easier engagement and retrieval of
retained catheter fragments in the vena cava. Commonly
used retrieval tools include Amplatz Goose Neck snare
(ev3 Inc, Plymouth [MN], US; Figure 5) or EN Snare
(Merit Medical, West Jordan [UT], US; Figure 6).
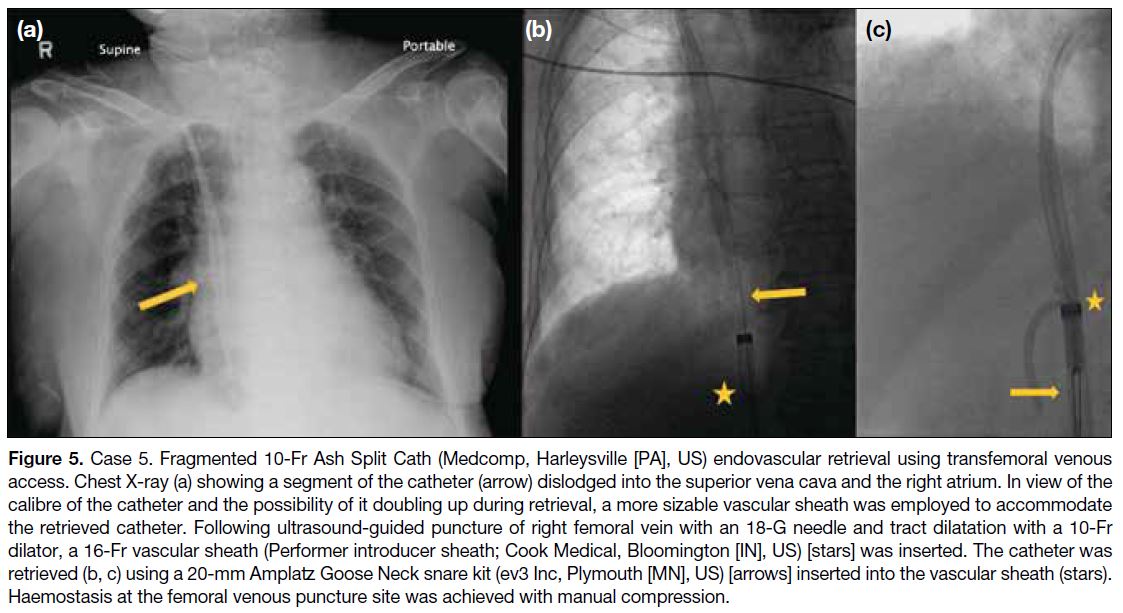
Figure 5. Case 5. Fragmented 10-Fr Ash Split Cath (Medcomp, Harleysville [PA], US) endovascular retrieval using transfemoral venous
access. Chest X-ray (a) showing a segment of the catheter (arrow) dislodged into the superior vena cava and the right atrium. In view of the
calibre of the catheter and the possibility of it doubling up during retrieval, a more sizable vascular sheath was employed to accommodate
the retrieved catheter. Following ultrasound-guided puncture of right femoral vein with an 18-G needle and tract dilatation with a 10-Fr
dilator, a 16-Fr vascular sheath (Performer introducer sheath; Cook Medical, Bloomington [IN], US) [stars] was inserted. The catheter was
retrieved (b, c) using a 20-mm Amplatz Goose Neck snare kit (ev3 Inc, Plymouth [MN], US) [arrows] inserted into the vascular sheath
(stars). Haemostasis at the femoral venous puncture site was achieved with manual compression.
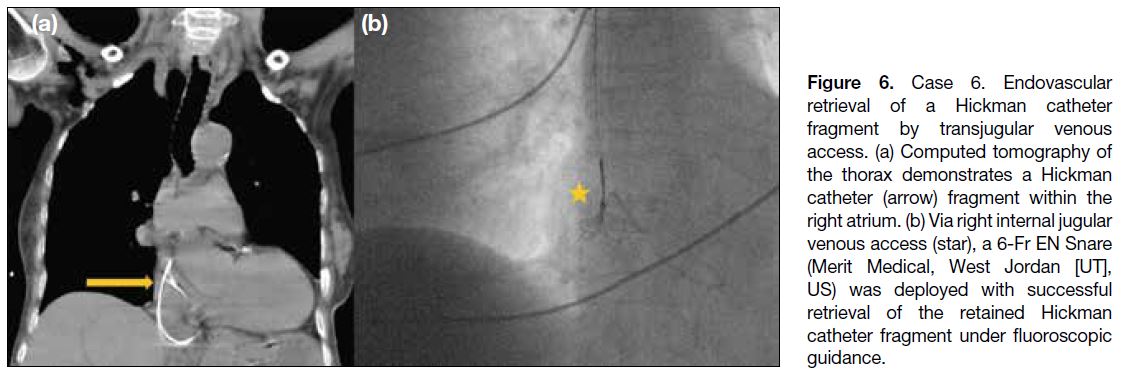
Figure 6. Case 6. Endovascular retrieval of a Hickman catheter fragment by transjugular venous access. (a) Computed tomography of the thorax demonstrates a Hickman catheter (arrow) fragment within the right atrium. (b) Via right internal jugular venous access (star), a 6-Fr EN Snare (Merit Medical, West Jordan [UT], US) was deployed with successful retrieval of the retained Hickman catheter fragment under fluoroscopic guidance.
FIBRIN SHEATH STRIPPING AROUND RETAINED CATHETER THROUGH TRANSFEMORAL VENOUS ACCESS
Fibrin sheath formation is a common complication with
long-term indwelling catheter, leading to encasement
of catheter tip or side hole impairing catheter patency,
thrombus formation or infective complications.[13] Fibrin
sheaths may also be adherent to the catheter and vessel
wall, precluding catheter removal. While fibrin sheath
detection from plain radiography or cross-sectional
imaging is difficult given their thin appearance,
fluoroscopy with contrast injection into the affected
catheter is helpful in depicting fibrin sheath as filling
defects, as well as contrast reflux along the proximal
catheter with efflux from defects in the sleeve, or
excessive ejection of contrast material from the side
holes of the proximal port,[13] which may be secondary to
blockage of catheter tip outflow by fibrin sheath.
In the event of adherent catheter to vessel wall due to
fibrin sheath precluding catheter removal, fibrin sheath stripping with snare-ride technique can be helpful in
achieving release and successful removal of the catheter
that was stuck to the vessel wall by adherent fibrin sheath
(Figure 7).
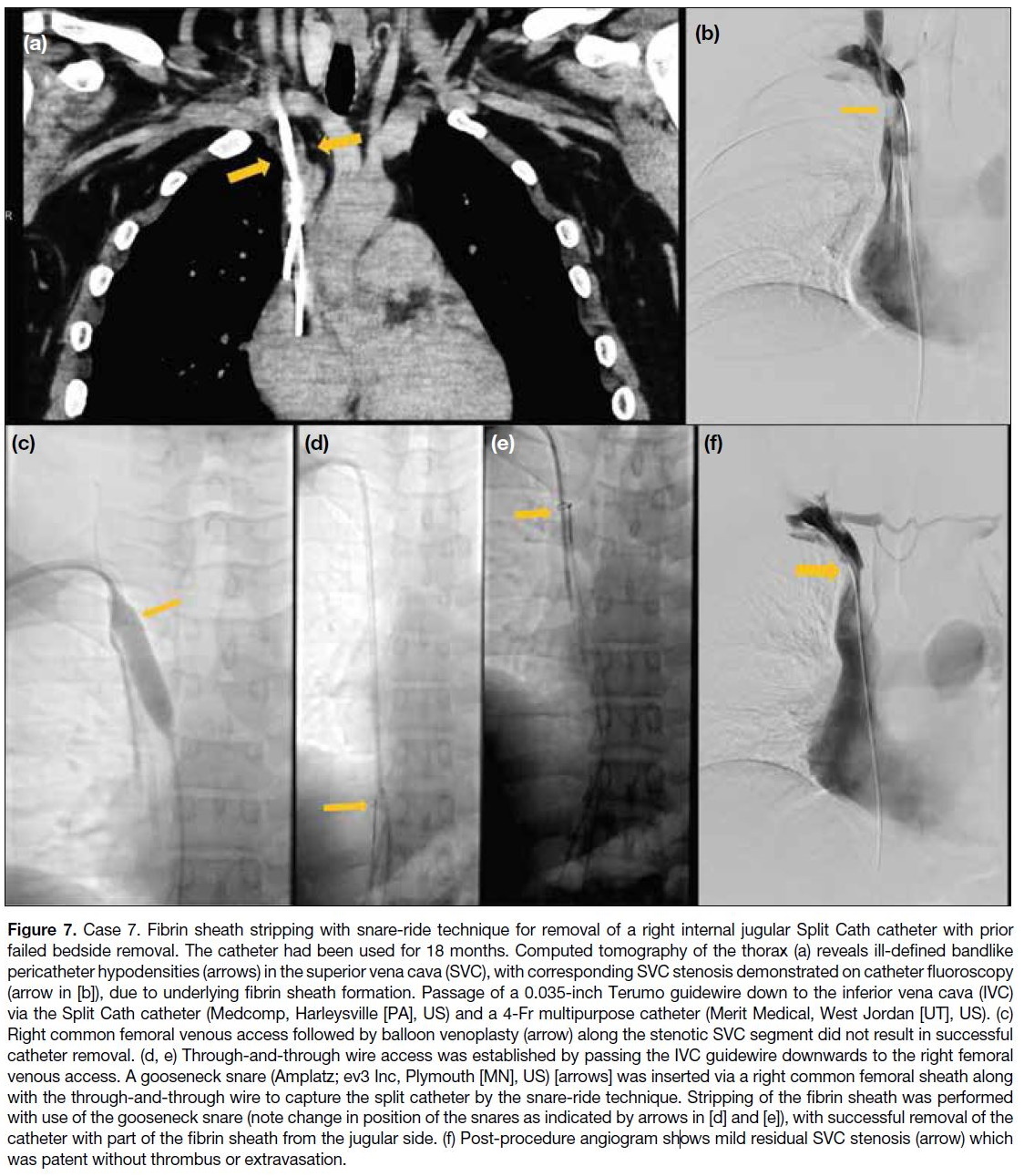
Figure 7. Case 7. Fibrin sheath stripping with snare-ride technique for removal of a right internal jugular Split Cath catheter with prior
failed bedside removal. The catheter had been used for 18 months. Computed tomography of the thorax (a) reveals ill-defined bandlike
pericatheter hypodensities (arrows) in the superior vena cava (SVC), with corresponding SVC stenosis demonstrated on catheter fluoroscopy
(arrow in [b]), due to underlying fibrin sheath formation. Passage of a 0.035-inch Terumo guidewire down to the inferior vena cava (IVC)
via the Split Cath catheter (Medcomp, Harleysville [PA], US) and a 4-Fr multipurpose catheter (Merit Medical, West Jordan [UT], US). (c)
Right common femoral venous access followed by balloon venoplasty (arrow) along the stenotic SVC segment did not result in successful
catheter removal. (d, e) Through-and-through wire access was established by passing the IVC guidewire downwards to the right femoral
venous access. A gooseneck snare (Amplatz; ev3 Inc, Plymouth [MN], US) [arrows] was inserted via a right common femoral sheath along
with the through-and-through wire to capture the split catheter by the snare-ride technique. Stripping of the fibrin sheath was performed
with use of the gooseneck snare (note change in position of the snares as indicated by arrows in [d] and [e]), with successful removal of the
catheter with part of the fibrin sheath from the jugular side. (f) Post-procedure angiogram shows mild residual SVC stenosis (arrow) which
was patent without thrombus or extravasation.
CONCLUSION
In the unfortunate event of iatrogenic vascular injury
during catheterisation, prompt assessment with computed
tomographic angiography to delineate the intravascular course of the malpositioned catheter, its relationship to
adjacent vital vasculature, and detection of complications
is crucial for treatment planning. Careful planning
of extraction routes and use of appropriate retrieval
devices for retained catheter components are pivotal.
Multidisciplinary collaboration, providing knowledge in
different interventional radiological treatment options,
can enable safe and effective intervention with reduced
patient morbidity and enhanced recovery.
REFERENCES
1. Saugel B, Scheeren TW, Teboul JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care. 2017;21:225. Crossref
2. Ben Mrad I, Ben Fatma L, Ben Mrad M, Miri R, Mleyhi S, Mami I, et al. Endovascular management of a subclavian arterial injury during central venous catheter placement for hemodialysis.
Open Access Emerg Med. 2021;13:273-7. Crossref
3. Pikwer A, Acosta S, Kölbel T, Malina M, Sonesson B, Akeson J. Management of inadvertent arterial catheterisation associated with central venous access procedures. Eur J Vasc Endovasc Surg. 2009;38:707-14. Crossref
4. Practice guidelines for central venous access 2020: an updated report by the American Society of Anesthesiologists Task Force on Central Venous Access [editorial]. Anesthesiology. 2020;132:8-43. Crossref
5. Guilbert MC, Elkouri S, Bracco D, Corriveau MM, Beaudoin N,
Dubois MJ, et al. Arterial trauma during central venous catheter
insertion: case series, review and proposed algorithm. J Vasc Surg.
2008;48:918-25; discussion 925. Crossref
6. Dornbos DL 3rd, Nimjee SM, Smith TP. Inadvertent arterial
placement of central venous catheters: systematic review and
guidelines for treatment. J Vasc Interv Radiol. 2019;30:1785-94. Crossref
7. Ng KT, Chau CM, Chan HF, Cheng LF, Ma KF, Chan KM. Percutaneous repair of inadvertent brachiocephalic arterial puncture by closure device: a case report. Hong Kong J Radiol. 2020;23:134-8. Crossref
8. Lorenzo JF, Rey JV, Arquillo IL, Encisa de Sá JM. Off-label use of ProGlide percutaneous closure device in iatrogenic arterial catheterizations: our experience. Vascular. 2020;28:756-9. Crossref
9. Sharma M, Sakhuja R, Teitel D, Boyle A. Percutaneous arterial closure for inadvertent cannulation of the subclavian artery—a call for caution. J Invasive Cardiol. 2008;20:E229-32.
10. Sarioglu O, Capar AE, Belet U. Interventional treatment options in pseudoaneurysms: different techniques in different localizations. Pol J Radiol. 2019;84:e319-27. Crossref
11. Lo AX, Hon TY, Luk WH, Loke TK, Lo SS, Chan JC. Ultrasound-guided thrombin injection for pseudoaneurysms: a case series at a local hospital. Hong Kong Med J. 2012;18:333-7.
12. Woodhouse JB, Uberoi R. Techniques for intravascular foreign body retrieval. Cardiovasc Intervent Radiol. 2013;36:888-97. Crossref
13. Faintuch S, Salazar GM. Malfunction of dialysis catheters: management of fibrin sheath and related problems. Tech Vasc Interv Radiol. 2008;11:195-200. Crossref