Early Local Community Data on Safety and Efficacy of Fruquintinib in Metastatic Colorectal Cancer
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2024 Sep;27(3):e147-55 | Epub 16 September 2024
Early Local Community Data on Safety and Efficacy of Fruquintinib in Metastatic Colorectal Cancer
HK So1, TTS Lau1, NSM Wong2, M Tong3, JJ Huang3, CY Shum1
1 Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
2 Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong SAR, China
3 Cluster Quality and Safety Division, Tuen Mun Hospital, Hong Kong SAR, China
Correspondence: Dr HK So, Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China. Email: hk.so@ha.org.hk
Submitted: 28 April 2024; Accepted: 2 July 2024.
Contributors: HKS, NSMW, TTSL and CYS designed the study. HKS, NSMW, MT and JJH acquired the data. HKS analysed the data and drafted the manuscript. NSMW, TTSL and CYS critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2023-006-2). Informed patient consent was waived by the Board due to the retrospective nature of the research and the use of anonymised data.
Abstract
Introduction
Fruquintinib, a selective inhibitor of vascular endothelial growth factor receptor-1, -2, and -3 tyrosine
kinases, is indicated for late-line treatment of metastatic colorectal cancer (mCRC). This retrospective study aimed to review the safety and efficacy of fruquintinib in the Hong Kong population.
Methods
Patients with mCRC who had failed at least two standard chemotherapy regimens were treated with
fruquintinib at two tertiary centres in Hong Kong between December 2021 and July 2023. We reported overall survival, event-free survival (EFS), disease control rate, and toxicity. EFS was defined as the time from starting treatment to an event, which could be disease progression, discontinuation of treatment for any reason, or death.
Results
A total of 26 mCRC patients were treated with fruquintinib. The median overall survival and median EFS
were 8.9 months and 4.2 months, respectively. Among the 22 patients who experienced an event, 15 (57.7%) had
disease progression, six (23.1%) discontinued treatment for any reason, and one (3.8%) died. The disease control
rate was 38.5%, including two (7.7%) patients with partial response and eight (30.8%) patients with stable disease.
Grade ≥3 adverse reactions occurred in 69.2% of patients, the most common of which were hypertension (53.8%),
hand-foot syndrome (19.2%), and diarrhoea (11.5%). There were no treatment-related deaths.
Conclusion
Fruquintinib demonstrated reasonable clinical efficacy and a manageable safety profile, consistent with
the findings of international clinical studies. It is a valid option for later-line mCRC patients.
Key Words: Carcinoembryonic antigen; ErbB receptors; Hand-foot syndrome; Vascular endothelial growth factor A
中文摘要
使用呋喹替尼治療轉移性大腸癌的安全性及有效性的早期本地社區數據
蘇衍錕、劉芷珊、黃善敏、唐雯、黃嘉杰、岑翠瑜
引言
呋喹替尼是一種血管內皮生長因子受體(VEGFR)-1、-2及-3酪胺酸激酶選擇性抑制劑,適用於轉移性大腸癌的後期治療。本回顧性研究旨在調查於香港人口使用呋喹替尼的安全性及有效性。
方法
經歷最少兩次標準化療方案失敗的轉移性大腸癌患者於2021年12月至2023年7月期間在香港兩所三級醫療機構接受呋喹替尼治療。我們報告整體存活期、無事件存活期、疾病控制率及毒性數據。無事件存活期的定義為開始治療起計至有事件發生的時間,可能包括病情惡化、因任何原因導致停止治療或死亡。
結果
共有26名轉移性大腸癌患者接受呋喹替尼治療。整體存活期中位數及無事件存活期中位數分別為8.9個月及4.2個月。在22名有事件發生的患者當中,15名(57.7%)病情惡化,6名(23.1%)因任何原因導致停止治療,1名(3.8%)死亡。疾病控制率為38.5%,包括兩名(7.7%)部分反應患者及8名(30.8%)反應穩定患者。共有69.2%患者出現三級或以上不良反應,最常見為高血壓(53.8%)、手足症候群(19.2%)及腹瀉(11.5%)。本研究沒有與治療有關的死亡個案。
結論
呋喹替尼具有合理的臨床有效性及易於管理的安全狀況,與國際臨床研究結果一致,是後期轉移性大腸癌患者的有效選項。
INTRODUCTION
Globally, colorectal cancer is the third most common
type of cancer and the second leading cause of cancer-related
deaths.[1] In Hong Kong, colorectal cancer was not
only the second most common cancer but also the second
most common cause of cancer-related deaths in 2020.[2]
The primary treatment for metastatic colorectal cancer
(mCRC) is chemotherapy, often supplemented by
targeted therapy and, in certain cases, immunotherapy
for patients with mismatch repair deficient tumours. For
chemotherapy, standard chemotherapy regimens include
5-fluorouracil (or its oral prodrug capecitabine) plus either
oxaliplatin or irinotecan, or both.[3] [4] Targeted therapy
agents include bevacizumab and aflibercept, which
target the vascular endothelial growth factor (VEGF)
pathway; and cetuximab and panitumumab, which target
the epidermal growth factor receptor (EGFR) pathway.[3] [4]
Later-line treatment regimens include trifluridine-tipiracil[5]
and regorafenib.[6] These two agents offer only
modest improvements in overall survival (OS) and
progression-free survival. However, even after failure on
multiple different treatment strategies, patients may still maintain good performance status. This underscores the
necessity for more safe and effective treatment options.
Fruquintinib is a selective inhibitor of vascular
endothelial growth factor receptor (VEGFR)-1, -2, and -3
tyrosine kinases.[7] In the phase III FRESCO randomised
clinical trial (Fruquintinib Efficacy and Safety in 3+ Line
Colorectal Cancer Patients), fruquintinib significantly
improved the median overall survival (mOS) compared
with that of the placebo group (9.3 months [95%
confidence interval (CI) = 8.2-10.5] vs. 6.6 months
[95% CI = 5.9-8.1]) in Chinese patients with mCRC
who progressed after at least two prior chemotherapy
regimens (i.e., third- or later-line use).[8] It was approved
in Mainland China in 2018 and was granted a fast-track
designation by the US Food and Drug Administration in
June 2020 for the above indication.[9] Another recent phase
III FRESCO-2 randomised clinical trial also showed
significant improvement in mOS with fruquintinib
compared with placebo (7.4 months [95% CI = 6.7-8.2] vs. 4.8 months [95% CI = 4.0-5.8]).[10] Fruquintinib
received its approval from the US Food and Drug
Administration on 8 November 2023, for adult patients with mCRC who had previously received 5-fluorouracil,
oxaliplatin and irinotecan-based chemotherapy, anti-VEGF therapy, and anti-EGFR therapy (if the tumour was RAS-wild type).[11]
The FRESCO trial recruited 416 Chinese patients from
Mainland China, where fruquintinib was developed.[8]
On the other hand, the FRESCO-2 trial included
patients from North America, Europe, Australia and
Japan, but Japanese patients comprised <10% of the
trial population.[10] The FRESCO trial excluded patients
who had been previously exposed to regorafenib,[8]
while patients who progressed on or were intolerant
to trifluridine-tipiracil or regorafenib could enter the
FRESCO-2 trial.[10] Hong Kong was not a study site in
either trial, and local experience in the use of fruquintinib
was scarce.
This study aimed to analyse the safety and efficacy
of fruquintinib in mCRC patients. To the best of our
knowledge, this is the first retrospective study of
fruquintinib in public healthcare setting in the local
population.
METHODS
Data Collection and Participants
Clinical data from 26 patients who received fruquintinib
between 31 December 2021 and 22 July 2023 were
retrospectively reviewed and collected from the
institutional databases of two tertiary centres in Hong
Kong, namely, Princess Margaret Hospital and Tuen
Mun Hospital. The inclusion criteria for fruquintinib
use were modified from the FRESCO[8] and FRESCO-2[10]
trials, which were as follows: (1) age ≥18 years; (2)
an Eastern Cooperative Oncology Group (ECOG)
performance status score of 0 to 1; (3) histologically
confirmed mCRC; (4) failure (progressive disease or
intolerance) on at least two standard chemotherapy
regimens using fluoropyrimidine, irinotecan, oxaliplatin,
anti-VEGF antibodies (bevacizumab and aflibercept),
or anti-EGFR antibodies (cetuximab or panitumumab);
(5) measurable disease by Response Evaluation Criteria
in Solid Tumors (RECIST) version 1.1; (6) adequate
bone marrow reserve (absolute neutrophil count ≥1.5
× 109/L, platelet count ≥100 × 109/L, and haemoglobin level ≥9.0 g/dL); (7) renal function (serum creatinine
level ≤1.5 × upper limit of normal [ULN] or creatinine
clearance ≥60 mL/min; urine dipstick protein of ≤1+ or
24-hour urine protein level <1.0 g/24 h); and (8) liver
function (serum total bilirubin level ≤1.5 × ULN; alanine
aminotransferase and aspartate aminotransferase level ≤2.5 × ULN in subjects without hepatic metastases; and
alanine aminotransferase and aspartate aminotransferase
level ≤5 × ULN in subjects with hepatic metastases).
There were no exclusion criteria involving prior use of
trifluridine-tipiracil or regorafenib.
Study Design
This was a local, single-arm, retrospective analysis of patients with mCRC conducted at two tertiary centres
in Hong Kong. These patients had either progressed
or shown intolerance after receiving at least two lines
of chemotherapy. The included patients underwent
repeated 28-day treatment cycles of fruquintinib, with
a schedule of 3 weeks on the medication (5 mg oral
daily) followed by a 1-week break. This treatment
cycle was continued until disease progression, death,
occurrence of unacceptable toxicity, or discontinuation
by the physician. Dose reduction was allowed to manage
treatment-related adverse effects and followed the
protocol of the FRESCO trial.[8]
Clinical Assessment Outcomes and Endpoints
The primary endpoint was OS, defined as the time from
the start of treatment using fruquintinib to death from
any cause. Tumour response assessment was performed
at intervals subject to the availability of imaging and
physician discretion, and response was defined by
RECIST version 1.1. The secondary endpoints were
event-free survival (EFS) [defined as the time from
starting treatment to an event, which could be disease
progression defined as the first documentation of disease
progression assessed by the investigator according to
RECIST version 1.1, discontinuation of treatment for
any reason, or death], duration of treatment (defined as
the time from starting treatment to last study treatment
dose), objective response rate (defined as confirmed
complete or partial response), disease control rate
(defined as the sum of the complete response, partial
response and stable disease rates), and carcinoembryonic
antigen (CEA) response. EFS was selected instead of
progression-free survival because the imaging intervals
in real-world settings vary. In heavily pretreated patients,
quality of life (QoL) is important, and discontinuation of
treatment for any reason can also indicate the tolerability
of a drug. For CEA response, a definition modified from
the RECIST criteria was used to evaluate treatment
response, and responses were classified into three groups,
namely, CEA-RD (responsive disease), CEA-SD (stable
disease), and CEA-PD (progressive disease).[12] [13] CEA-RD
was defined as a decrease of >30% from the original level; CEA-PD was defined as an increase of >20% from
the original level.[12] [13] A change in the CEA level that
did not meet the criteria for CEA-RD and CEA-PD was
defined as CEA-SD.[12] [13]
Adverse events (AEs) were recorded throughout the
study from the start of treatment to the end of the study
period or the start of the next line of treatment. They
were graded according to the National Cancer Institute
Common Terminology Criteria for Adverse Events
version 5.0.[14]
Statistical Analysis
For OS and EFS, the Kaplan-Meier method was used
to estimate the median survival time and 95% CI.
Relationships between individual patient characteristics
and OS or EFS were analysed using the Cox proportional
hazards model to estimate hazard ratios (HRs) and 95%
CIs. All analyses were performed using commercial
software SPSS (Windows version 28.0; IBM Corp,
Armonk [NY], US). A p value of < 0.05 was considered
statistically significant.
RESULTS
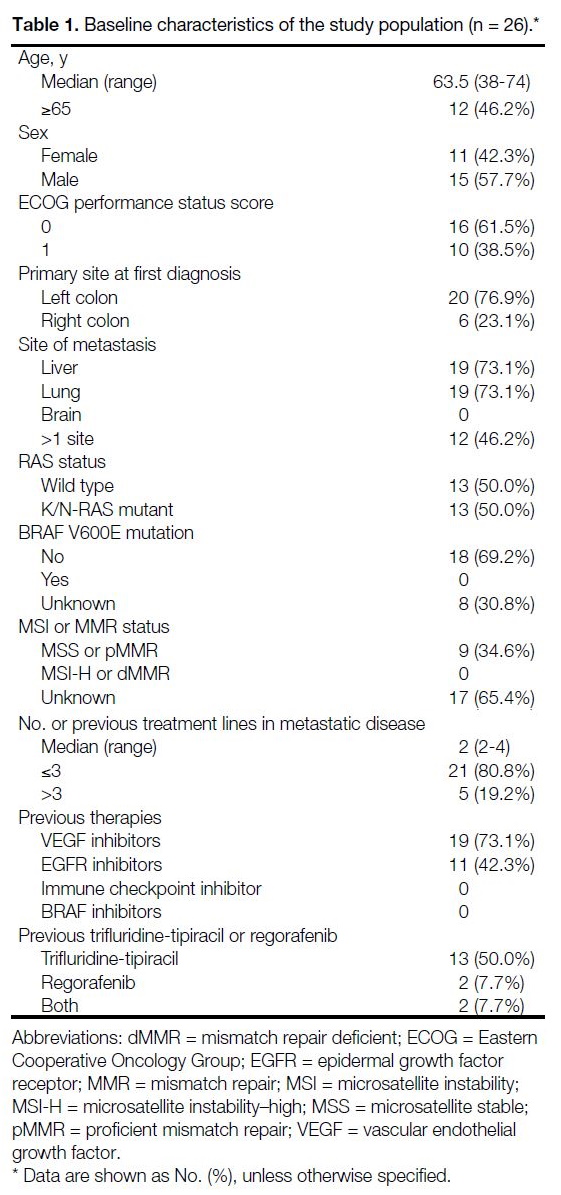
The baseline demographics and disease characteristics of the patients are shown in Table 1.
Table 1. Baseline characteristics of the study population (n = 26).
Efficacy
Survival Outcomes and Duration of Treatment
The median duration of treatment was 4.3 months (range, 0.6-15.4) and the median number of treatment cycles
was 3 (range, 1-17). The median follow-up time was 7.3
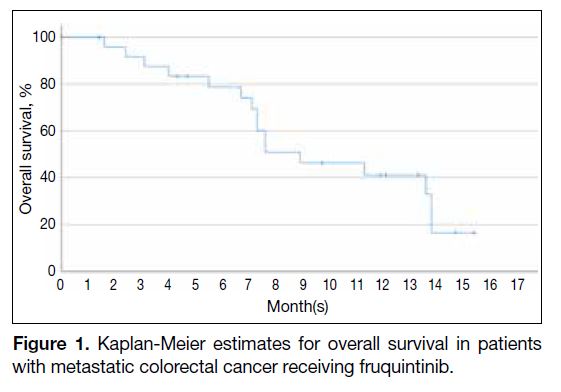
months. The mOS was 8.9 months (95% CI = 4.5-13.3).
The Kaplan-Meier plot for OS is shown in Figure 1. The
proportion of patients still alive at 6 months was 78.7%
and that at 12 months was 41.2%.
Figure 1. Kaplan-Meier estimates for overall survival in patients with metastatic colorectal cancer receiving fruquintinib.
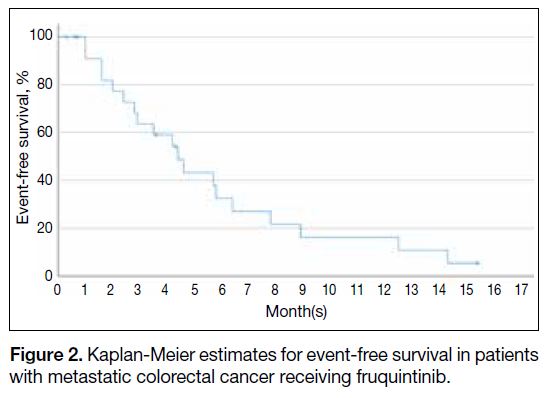
The median EFS was 4.2 months (95% CI = 2.5-5.9).
Among the 22 of 26 patients who experienced an
event, 15 (57.7%) had disease progression, six (23.1%)
discontinued treatment for any reason, and one (3.8%)
died. The Kaplan-Meier plot for EFS is shown in Figure 2.
Figure 2. Kaplan-Meier estimates for event-free survival in patients with metastatic colorectal cancer receiving fruquintinib.
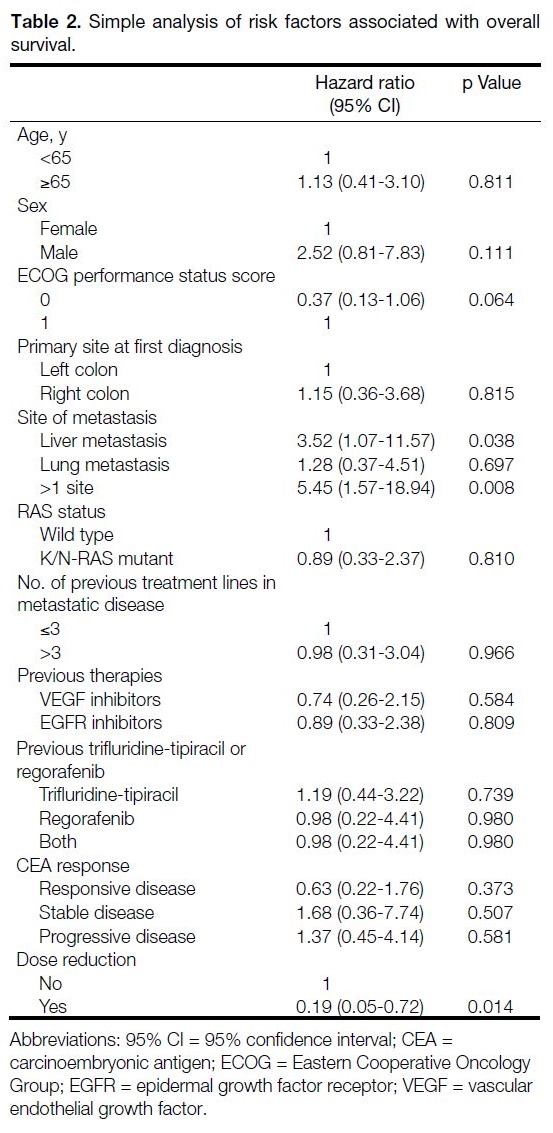
Subgroup analyses of OS and EFS were carried out
with a Cox proportional hazards model (simple and
multivariable), but only a few clinical, tumour, or
treatment factors exhibited a statistically significant
correlation (Tables 2, 3 and 4). Simple analysis also revealed
that OS was worse for patients with liver metastasis and
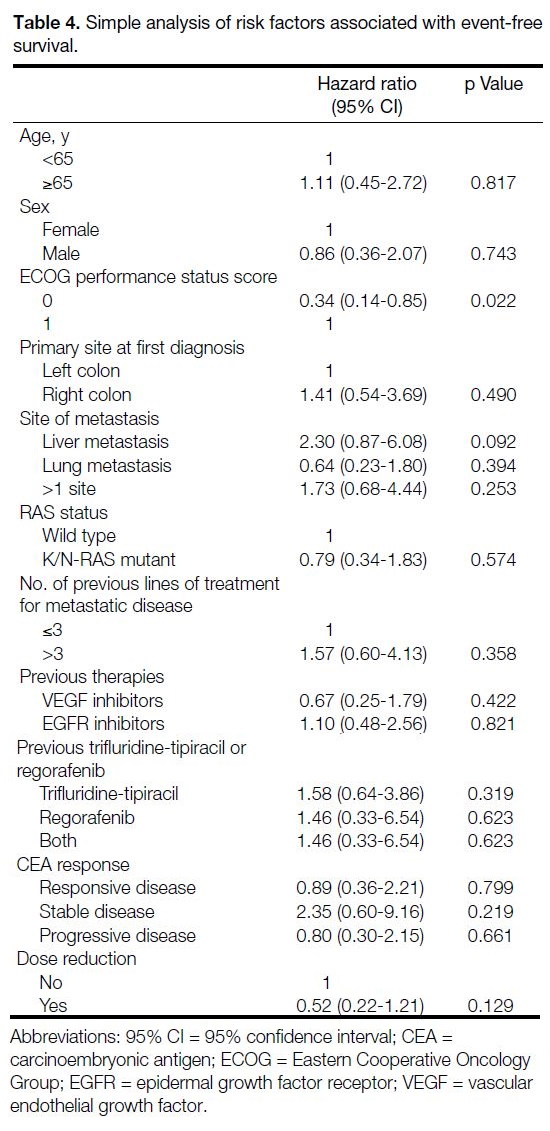
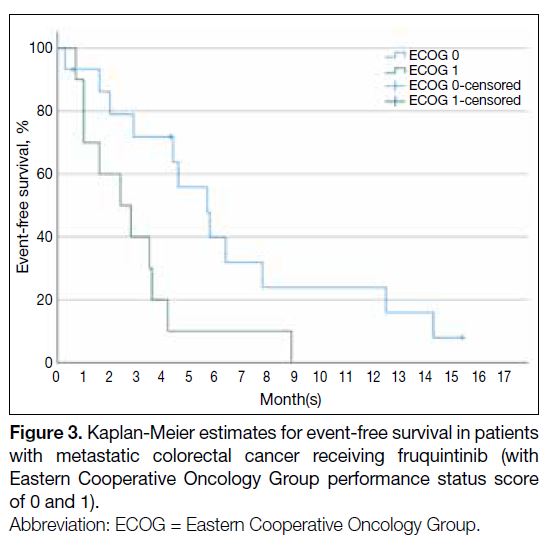
with multiple sites of metastasis (Table 2). Patients with an ECOG performance status score of 0 had better EFS
(HR = 0.34; 95% CI = 0.14-0.85) [Table 4 and Figure 3].
Previous use of trifluridine-tipiracil, regorafenib, or both
did not significantly affect OS or EFS (Tables 2, 3 and 4).
Table 2. Simple analysis of risk factors associated with overall survival.
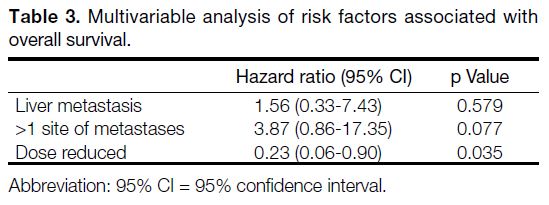
Table 3. Multivariable analysis of risk factors associated with overall survival.
Table 4. Simple analysis of risk factors associated with event-free survival.
Figure 3. Kaplan-Meier estimates for event-free survival in patients with metastatic colorectal cancer receiving fruquintinib (with Eastern Cooperative Oncology Group performance status score of 0 and 1).
The starting dose of fruquintinib was 5 mg daily (3 weeks on, 1 week off). 11 patients had their dose reduced, with
5 patients (19.2%) being reduced to 4 mg dailiy and 6 patients (23.1%) being reduced to 3 mg dailiy. Dose
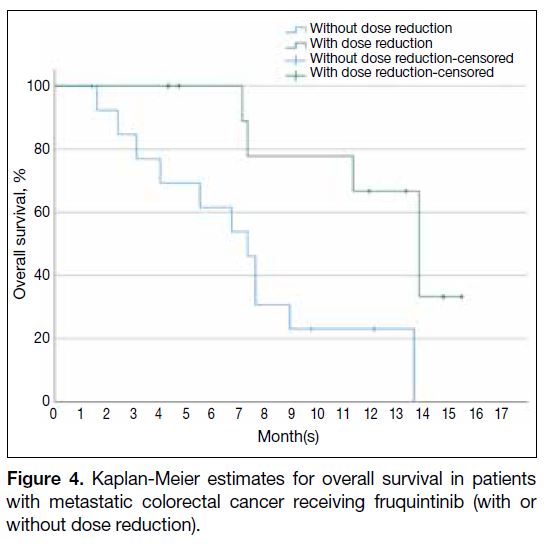
reduction of fruquintinib was associated with better OS
(HR = 0.19, 95% CI = 0.05-0.72; p = 0.014) [Table 2 and Figure 4], but not in EFS (HR = 0.52, 95% CI = 0.22-1.21; p = 0.129) [Table 4].
Figure 4. Kaplan-Meier estimates for overall survival in patients with metastatic colorectal cancer receiving fruquintinib (with or without dose reduction).
Radiological Response
In patients treated with fruquintinib, the disease control rate was 38.5% (10 of 26 patients), which included two
(7.7%) patients with partial response and eight patients
(30.8%) with stable disease. There was no patient with
complete response.
Carcinoembryonic Antigen Response
Regarding serum CEA responses, the differences in
OS and EFS were not statistically significant between
patients with CEA-RD, CEA-SD and CEA-PD.
Numerically, patients with CEA-RD had better OS and
EFS than patients with CEA-PD (Tables 2, 3 and 4).
Adverse Events
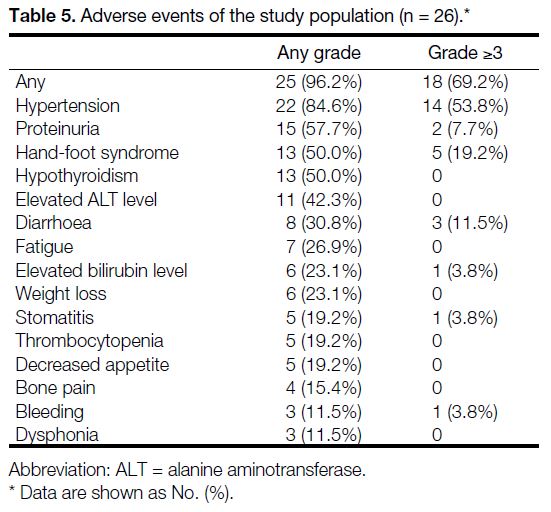
Twenty-five of 26 patients (96.2%) had at least one AE
of any grade (Table 5). The most frequently reported AEs of any grade were hypertension (84.6%), proteinuria
(57.7%), hand-foot syndrome (HFS) [50%], and
hypothyroidism (50%). Severe AEs (grade ≥3) occurred
in 18 patients (69.2%), with the most common being
hypertension (53.8%), HFS (19.2%), and diarrhoea
(11.5%) [Table 5]. There were no treatment-related
deaths in the study population.
Table 5. Adverse events of the study population (n = 26).
Six of 26 patients (23.1%) discontinued fruquintinib due to treatment-related AEs. The most frequent AE that led
to treatment discontinuation was HFS in two patients (7.7%). Treatment interruption due to AEs occurred
in five patients (19.2%), and the most common AE
associated with treatment interruption was hypertension
in two patients (7.7%). Dose reduction due to AEs
occurred in 11 patients (42.3%). The most frequent
AEs leading to dose reductions were HFS (11.5%),
proteinuria (11.5%), and diarrhoea (7.7%).
DISCUSSION
This retrospective study investigated the local
population treated with fruquintinib at two tertiary institutions in Hong Kong, which included patients who
had experienced disease progression following at least
two lines of chemotherapy. This study did not have a
placebo arm; the analysis of the results focuses on early
experience of safety and efficacy in our locality.
Efficacy
In this study, the mOS was 8.9 months and the median
EFS was 4.2 months, with a disease control rate of 38.5%.
In Hong Kong, third-line or beyond monotherapy options
for mCRC include trifluridine-tipiracil and regorafenib.
In the RECOURSE trial (trifluridine-tipiracil vs. placebo
in patients with previously treated mCRC), the treatment
group had an mOS of 7.1 months and a disease control
rate of 44%.[5] In the CONCUR trial (regorafenib vs.
placebo in Asian patients with previously treated
mCRC), the treatment group had an mOS of 8.8 months
and a disease control rate of 51%.[6] Our data suggest
that fruquintinib is a feasible monotherapy option in the
third line and beyond setting for mCRC patients in Hong
Kong.
OS and EFS analyses did not reveal any statistically
significant differences in most of the subgroups (Tables 2, 3 and 4), although OS was shown to worsen when
patients had liver metastasis or more than one site of
metastasis (Table 2). EFS was shown to be related to
ECOG performance status score (Table 4). There was
no statistically significant correlation between CEA
response and OS or EFS (Tables 2, 3 and 4), although OS
tended to improve in patients with CEA-RD and tended
to worsen in patients with CEA-PD.
In terms of the radiological and CEA (tumour marker)
response, there were more patients with CEA-RD than
there were with an objective response. This could be due
to less intensive imaging schedules in public hospital
settings, such that metabolic or relatively short-lived
treatment responses could not be captured radiologically.
Further study is needed to confirm the correlation
between CEA response and survival, and studying
the CEA response could help determine whether it
can supplement the suboptimal scanning schedule in
assessing the treatment response.
Adverse Events
The incidence of AEs and serious AEs was considerably
high in the study population. The most frequently reported
grade ≥3 AEs were hypertension, HFS, and diarrhoea
(Table 5). These AEs were manageable by supportive
measures and dose modification. The discontinuation of
fruquintinib in this study was 26.9%, whereas the rate
in FRESCO and FRESCO-2 were 15.1%[8] and 20%,[10]
respectively. Further QoL analysis would be valuable to
correlate the relatively high incidence of AEs and their
impact on patient’s QoL.
Baseline hypertension was a strong risk factor for high-grade hypertensive toxicity. Among the 14 patients who
experienced grade ≥3 hypertension, all had preexisting
hypertension at baseline. The baseline hypertension rate
(88.4%, n = 23) was relatively high when compared
to that of the general population (57.4% in the 65-84
age-group).[15] The odds ratio associated with grade
≥3 hypertension was 7.00 for patients with baseline
hypertension of any grade (95% CI = 0.34-144.06; p = 0.20). Of those patients with baseline hypertension,
only eight (34.8%) received intervention. Home blood
pressure monitoring was started or ensured in seven
patients, while antihypertensive therapy was started or
titrated only in one patient. Of the eight patients who
underwent intervention, seven (87.5%) still developed
grade ≥3 hypertension. These findings suggested that
more aggressive intervention by managing oncologists
is needed for patients with baseline hypertension.
According to the evidence from regorafenib, which is
also a VEGFR inhibitor, when encountering grade 2
hypertension, treating physicians can consider starting
a single antihypertensive agent (such as an angiotensin-converting
enzyme inhibitor).[16] For grade 3 hypertension,
an additional agent (such as a beta blocker) should be
considered, and if it remains refractory, a third agent
(such as a calcium channel blocker) may be added. Diuretics should be avoided because diarrhoea is also a common side-effect of fruquintinib, which may cause dehydration.
Another important adverse reaction was HFS. In HFS
management, preventive measures include reducing skin
friction, reducing exposure to heat, using skin barriers
and early identification of skin abrasions.[17] The use of
urea-based cream in combination with sorafenib (also
a VEGFR inhibitor) has been shown to reduce the
incidence of HFS.[18] Other commonly used measures
include analgesics, topical anaesthetics, topical high-potency
corticosteroids, keratolytic, and emollients.[17] If
the above supportive measures are not able to improve
tolerance, physicians can consider reducing the dose
according to the drug’s prescription information.
A total of 99% of the patients experienced any grade of AE in the fruquintinib (FRESCO-2) trial,[10] 97% in the
regorafenib (CONCUR) trial[6] and 98% in the trifluridinetipiracil
(RECOURSE) trial,[5] with 63%, 54%, and 69%
of patients experiencing grade ≥3 AEs, respectively. For
specific grade ≥3 AEs, we compared the same class of
drugs (i.e., fruquintinib vs. regorafenib), and the two drugs
had similar severe AE profiles. Compared with drugs of a
different class (i.e., fruquintinib vs. trifluridine-tipiracil),
the toxicity profiles of these agents clearly differed, with
the chemotherapy class (trifluridine-tipiracil) having
more haematological toxicity, as one would expect. The
most common severe AEs associated with trifluridine-tipiracil
were neutropenia (38%), leukopenia (21%), and
anaemia (18%).[5]
Patients in whom the dose was reduced had better OS
and tended to improve EFS. As the dose reduction was
mainly in response to toxicity, toxicity may be a predictor
of the VEGFR inhibitor treatment response. A similar
phenomenon was observed with anti-EGFR therapy, and
a worse skin reaction was proven to be associated with
a better response. In the OPUS study (untreated EGFR-expressing
advanced colorectal cancer, FOLFOX4 vs.
FOLFOX plus cetuximab),19 patients with grade 3 to 4
skin toxicity had a 66.7% response rate, while grade 1
patients and grade 0 patients had response rates of 42.2%
and 13%, respectively. In the EPIC study (The European
Prospective Investigation into Cancer and Nutrition,
second-line treatment after oxaliplatin-based therapy,
cetuximab plus irinotecan versus irinotecan),20 the
median survival was 5.8 months for grade 0 toxicities,
11.7 months for grade 1 to 2 toxicities, and 15.6 months
for grade 3 to 4 toxicities. However, further studies are needed to confirm this hypothesis in fruquintinib therapy.
Limitations
This study has several limitations. First, this was a
retrospective study based only on data from two public
tertiary centres in Hong Kong. Second, the sample
size was small, and some subgroup analyses did not
show statistical significance. Third, QoL data were not
collected in this study.
CONCLUSION
Fruquintinib demonstrated reasonable clinical efficacy
and a manageable safety profile and is a valid option
for later-line mCRC patients. Hypertension is the
most common high-grade toxicity, and preexisting
hypertension is a strong risk factor. Proactive
management of hypertension is strongly advocated.
Prompt AE management can optimise its clinical utility,
and dose reduction did not compromise efficacy. Further
study of treatment sequence and patient QoL among the
approved third-line or beyond options is needed.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49. Crossref
2. Hong Kong Cancer Registry, Hospital Authority. Top ten cancers. 2021. Available from: https://www3.ha.org.hk/cancereg/topten.html. Accessed 14 May 2024.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer, version 3. 2023. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 23 Sep 2023.
4. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10-32. Crossref
5. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-19. Crossref
6. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus
best supportive care versus placebo plus best supportive care in
Asian patients with previously treated metastatic colorectal cancer
(CONCUR): a randomised, double-blind, placebo-controlled, phase
3 trial. Lancet Oncol. 2015;16:619-29. Crossref
7. Cao J, Zhang J, Peng W, Chen Z, Fan S, Su W, et al. A phase I study
of safety and pharmacokinetics of fruquintinib, a novel selective
inhibitor of vascular endothelial growth factor receptor-1, -2, and-3
tyrosine kinases in Chinese patients with advanced solid tumors.
Cancer Chemother Pharmacol. 2016;78:259-69. Crossref
8. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib
vs placebo on overall survival in patients with previously treated
metastatic colorectal cancer: the FRESCO randomized clinical trial.
JAMA. 2018;319:2486-96. Crossref
9. HUTCHMED. Chi-Med announces fruquintinib granted U.S. FDA fast track designation for metastatic colorectal cancer [Press Release]. 2020 June 18. Available from: https://www.hutch-med.com/fruquintinib-granted-us-fda-fast-track-designation-for-mcrc/. Accessed 2 Sep 2024.
10. Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an
international, multicentre, randomised, double-blind, phase 3 study.
Lancet. 2023;402:41-53. Crossref
11. United States Food and Drug Administration. FDA approves fruquintinib in refractory metastatic colorectal cancer. 2023 November 8. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fruquintinib-refractory-metastatic-colorectal-cancer. Accessed 2 Sep 2024.
12. Su YT, Chen JW, Chang SC, Jiang JK, Huang SC. The clinical experience of the prognosis in opposite CEA and image change after therapy in stage IV colorectal cancer. Sci Rep. 2022;12:20075. Crossref
13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-47. Crossref
14. United States Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017 November 27. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed 2 Sep 2024.
15. Centre for Health Protection, Department of Health, Hong Kong SAR Government. Hypertension. May 2023. Available from: https://www.chp.gov.hk/en/healthtopics/content/25/35390.html. Accessed 20 Jan 2024.
16. Krishnamoorthy SK, Relias V, Sebastian S, Jayaraman V, Saif MW. Management of regorafenib-related toxicities: a review. Therap Adv Gastroenterol. 2015;8:285-97. Crossref
17. Kwakman JJ, Elshot YS, Punt CJ, Koopman M. Management of cytotoxic chemotherapy-induced hand-foot syndrome. Oncol Rev. 2020;14:442. Crossref
18. Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, et al. Randomized
controlled trial of the prophylactic effect of urea-based cream
on sorafenib-associated hand-foot skin reactions in patients with
advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:894-900. Crossref
19. Bokemeyer C, Bondarenko I, Makhson A, et al. Cetuximab plus
5-FU/FA/oxaliplatin (FOLFOX-4) versus FOLFOX-4 in the first-line
treatment of metastatic colorectal cancer (mCRC): OPUS, a
randomized phase II study. J Clin Oncol. 2007;25(Suppl 18):4035. Crossref
20. Jonker DJ, Karapetis CS, Moore M, Zalcberg JR, Tu D, Berry S, et al.
editors. Randomized phase III trial of cetuximab monotherapy
plus best supportive care (BSC) versus BSC alone in patients with
pretreated metastatic epidermal growth factor receptor (EGFR)–positive colorectal carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) and the
Australasian Gastro-Intestinal Trials Group (AGITG). In: Proc
Am Assoc Cancer Res; 2007.