Endovascular Management of Renal Arteriovenous Fistula: Three Case Reports
CASE REPORT
Endovascular Management of Renal Arteriovenous Fistula: Three Case Reports
JK Fung, HK Chin, WKW Leung, KYK Tang, CY Chu, WK Kan
Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr JK Fung, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email:
Submitted: 2 September 2023; Accepted: 18 July 2024.
Contributors: All authors designed the study. HKC, KYKT and CYC acquired the data. JKF, HKC, WKWL, KYKT and CYC analysed the data. JKF, HKC and WKWL drafted the manuscript. JKF, HKC, WKWL, KYKT and WKK critically revised the manuscript for important intellectual
content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-080-5). The requirement for informed patient consent was waived by the Board due to the retrospective nature of the study.
INTRODUCTION
Renal arteriovenous fistula (AVF) is a rare vascular
anomaly classified as traumatic or non-traumatic.
There are no guidelines for endovascular treatment.
Some case reports involve coil deployment[1] [2] but some
require additional techniques such as vascular plugs,
occlusive balloons, or stents[3] [4] to minimise the risk of
coil embolisation.
We report three cases of renal AVF endovascular
treatment, including two idiopathic AVFs, and focus on
treatment considerations and technical perspectives with
reference to current reported practices.
CASE PRESENTATIONS
Case 1
A 68-year-old female presented with haematuria. Elective
computed tomography (CT) urogram demonstrated an
AVF in the left kidney at the mid to lower pole. It was
supplied by a hypertrophied renal artery, drained by a
dilated renal vein and via the engorged inferior vena
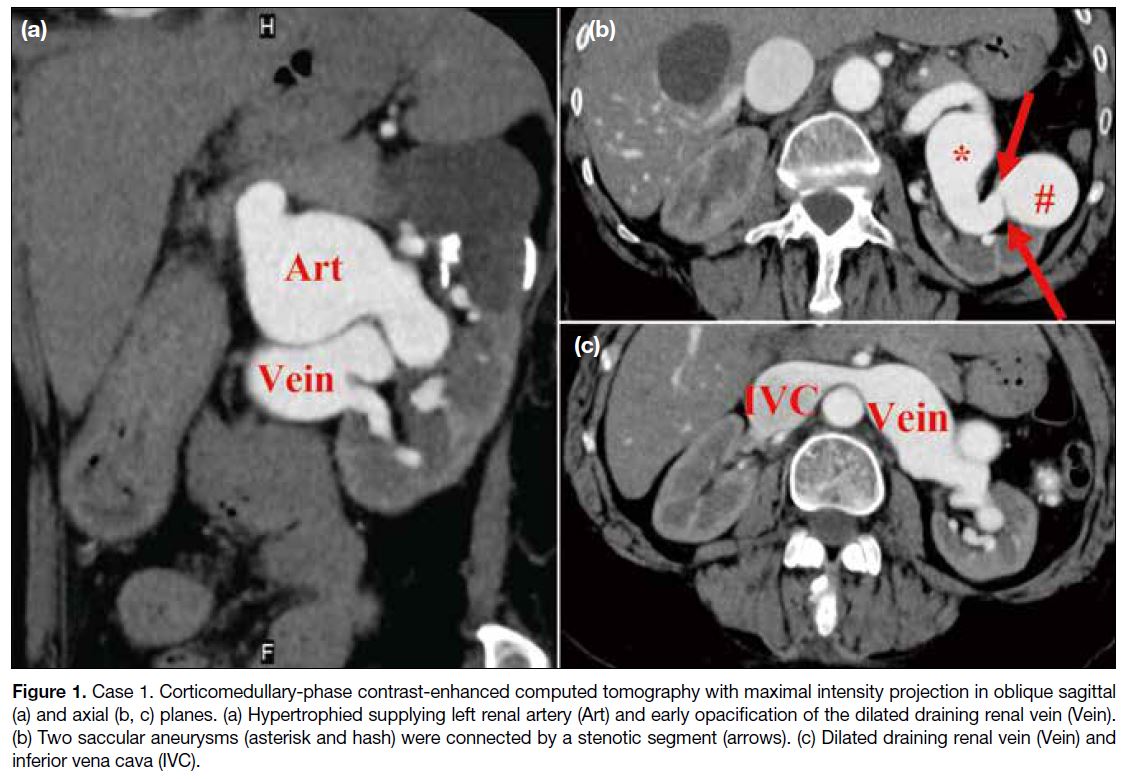
cava into the enlarged right atrium (Figure 1a and c).
There were two relatively sizeable saccular aneurysms
connected by a short stenotic segment (Figure 1b). The case was discussed with the vascular team and deemed
unsuitable for endovascular stenting.
Figure 1. Case 1. Corticomedullary-phase contrast-enhanced computed tomography with maximal intensity projection in oblique sagittal
(a) and axial (b, c) planes. (a) Hypertrophied supplying left renal artery (Art) and early opacification of the dilated draining renal vein (Vein).
(b) Two saccular aneurysms (asterisk and hash) were connected by a stenotic segment (arrows). (c) Dilated draining renal vein (Vein) and
inferior vena cava (IVC).
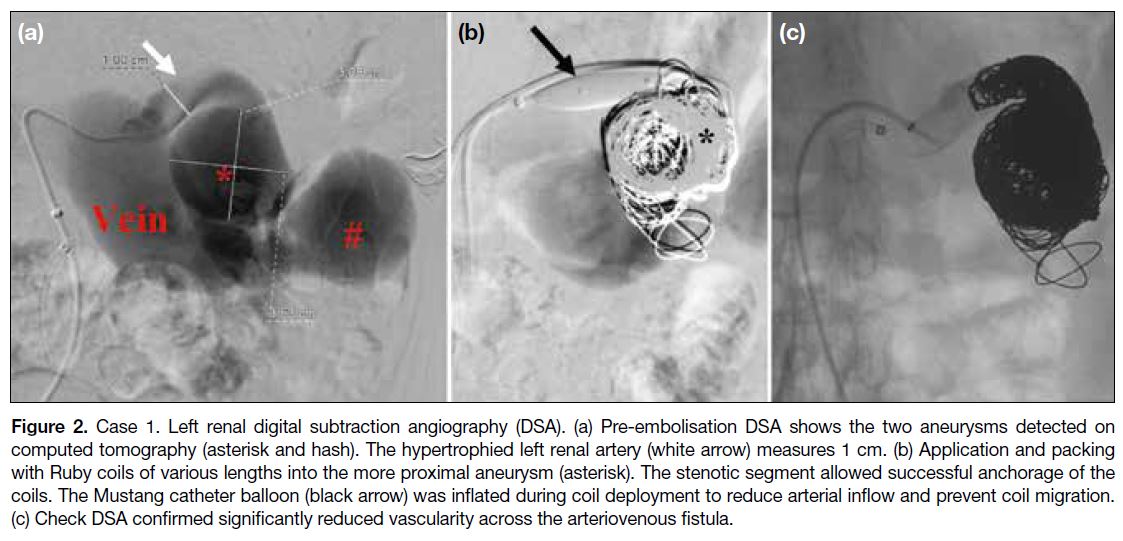
Digital subtraction angiography (DSA) confirmed the
AVF was supplied by the main trunk of the left renal
artery. The most proximal aneurysm was the largest at
3.5 cm (Figure 2a). Two 6-Fr guiding sheaths (Flexor
Ansel; Cook Medical, Bloomington [IN], US) were
advanced to the left main renal artery via a femoral
approach. A 0.035-inch balloon catheter (Mustang
[10 × 20 mm]; Boston Scientific, Marlborough [MA],
US) was then directed to the proximal left main renal
artery to control arterial inflow. Detachable coils
(standard Ruby coils; Penumbra Inc, Alameda [CA],
US) of various sizes and lengths were deployed into the
most proximal aneurysm using the scaffold technique
via a dedicated microcatheter (Excelsior XT-27; Stryker,
Kalamazoo [MI], US) [Figure 2b]. The feeding left
renal artery was eventually packed with detachable coils
(0.035-inch Interlock; Boston Scientific, Marlborough
[MA], US). Follow-up magnetic resonance (MR) renal
angiogram 6 months later revealed significantly reduced
vascularity in the dilated vessels and aneurysms (Figure 2c).
Figure 2. Case 1. Left renal digital subtraction angiography (DSA). (a) Pre-embolisation DSA shows the two aneurysms detected on
computed tomography (asterisk and hash). The hypertrophied left renal artery (white arrow) measures 1 cm. (b) Application and packing
with Ruby coils of various lengths into the more proximal aneurysm (asterisk). The stenotic segment allowed successful anchorage of the
coils. The Mustang catheter balloon (black arrow) was inflated during coil deployment to reduce arterial inflow and prevent coil migration.
(c) Check DSA confirmed significantly reduced vascularity across the arteriovenous fistula.
Case 2
A 49-year-old female presented with gross haematuria
and loss of consciousness. Haemoglobin level was
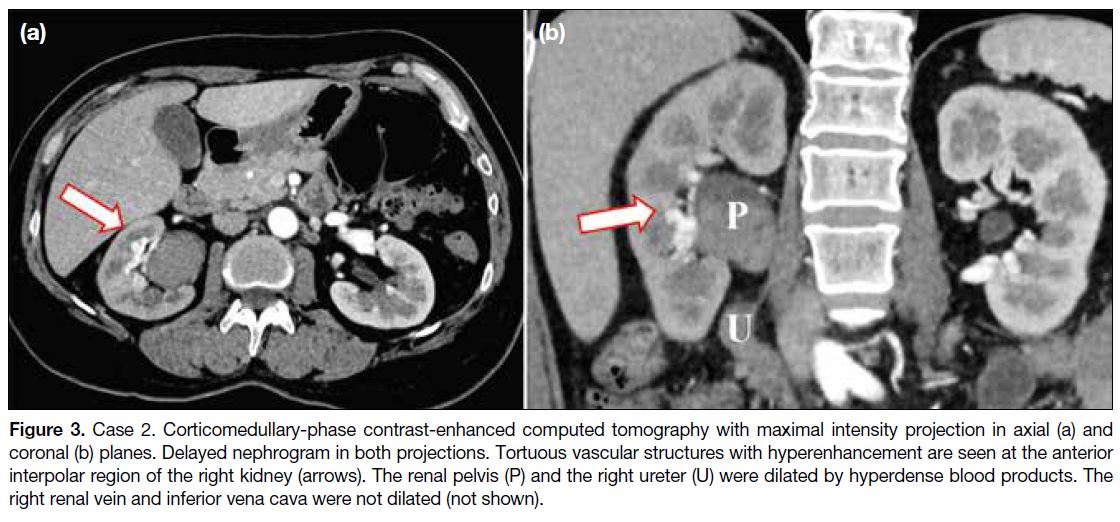
7.8 g/dL on admission. CT urogram demonstrated a right
AVF centred at the interpolar region, with acute blood products dilating the right renal collecting system and
the right ureter (Figure 3).
Figure 3. Case 2. Corticomedullary-phase contrast-enhanced computed tomography with maximal intensity projection in axial (a) and
coronal (b) planes. Delayed nephrogram in both projections. Tortuous vascular structures with hyperenhancement are seen at the anterior
interpolar region of the right kidney (arrows). The renal pelvis (P) and the right ureter (U) were dilated by hyperdense blood products. The
right renal vein and inferior vena cava were not dilated (not shown).
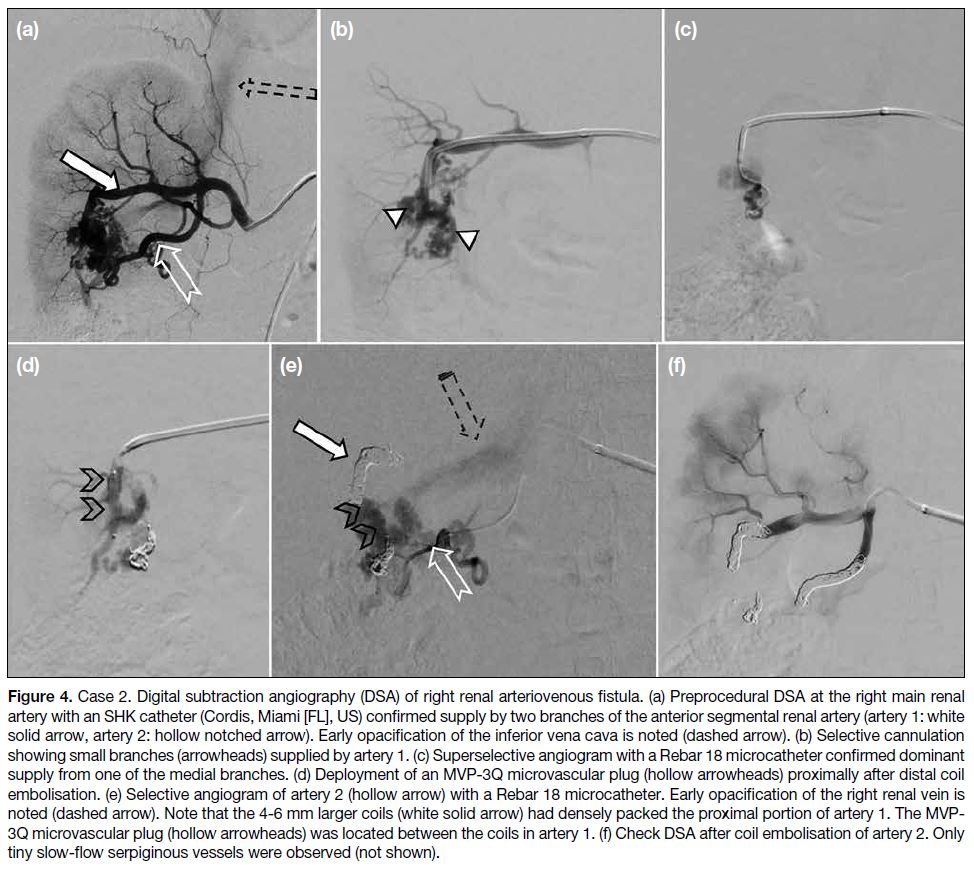
On DSA, the AVF was shown to be supplied by two
branches from the anterior segmental renal artery (Figure 4a). Selective cannulation was achieved with a 2.7-Fr microcatheter (Rebar 18 reinforced microcatheter;
Medtronic, Minneapolis [MN], US) [Figure 4c]. The
first vessel supplying the right AVF was embolised with
two detachable coils (Concerto Helix coils; Medtronic,
Minneapolis [MN], US). A microvascular plug (MVP-3Q; Medtronic, Minneapolis [MN], US) was launched
more proximally (Figure 4d) along the first artery to
effectively address the smaller side branches (Figure 4d). The remaining artery was packed with detachable
coils. The second artery supplying the AVF was first
embolised with a detachable coil, followed by pushable
coils (Nester microcoils; Cook Medical, Bloomington
[IN], US) [Figure 4e]. Repeat DSA confirmed significant
reduction in AVF vascularity [Figure 4f]. There was no
recurrence of haematuria at 1-year clinical follow-up.
Figure 4. Case 2. Digital subtraction angiography (DSA) of right renal arteriovenous fistula. (a) Preprocedural DSA at the right main renal
artery with an SHK catheter (Cordis, Miami [FL], US) confirmed supply by two branches of the anterior segmental renal artery (artery 1: white
solid arrow, artery 2: hollow notched arrow). Early opacification of the inferior vena cava is noted (dashed arrow). (b) Selective cannulation
showing small branches (arrowheads) supplied by artery 1. (c) Superselective angiogram with a Rebar 18 microcatheter confirmed dominant
supply from one of the medial branches. (d) Deployment of an MVP-3Q microvascular plug (hollow arrowheads) proximally after distal coil
embolisation. (e) Selective angiogram of artery 2 (hollow arrow) with a Rebar 18 microcatheter. Early opacification of the right renal vein is
noted (dashed arrow). Note that the 4-6 mm larger coils (white solid arrow) had densely packed the proximal portion of artery 1. The MVP-3Q microvascular plug (hollow arrowheads) was located between the coils in artery 1. (f) Check DSA after coil embolisation of artery 2. Only
tiny slow-flow serpiginous vessels were observed (not shown).
Case 3
A 48-year-old man was diagnosed with end-stage renal
failure and managed by haemodialysis. A left renal AVF
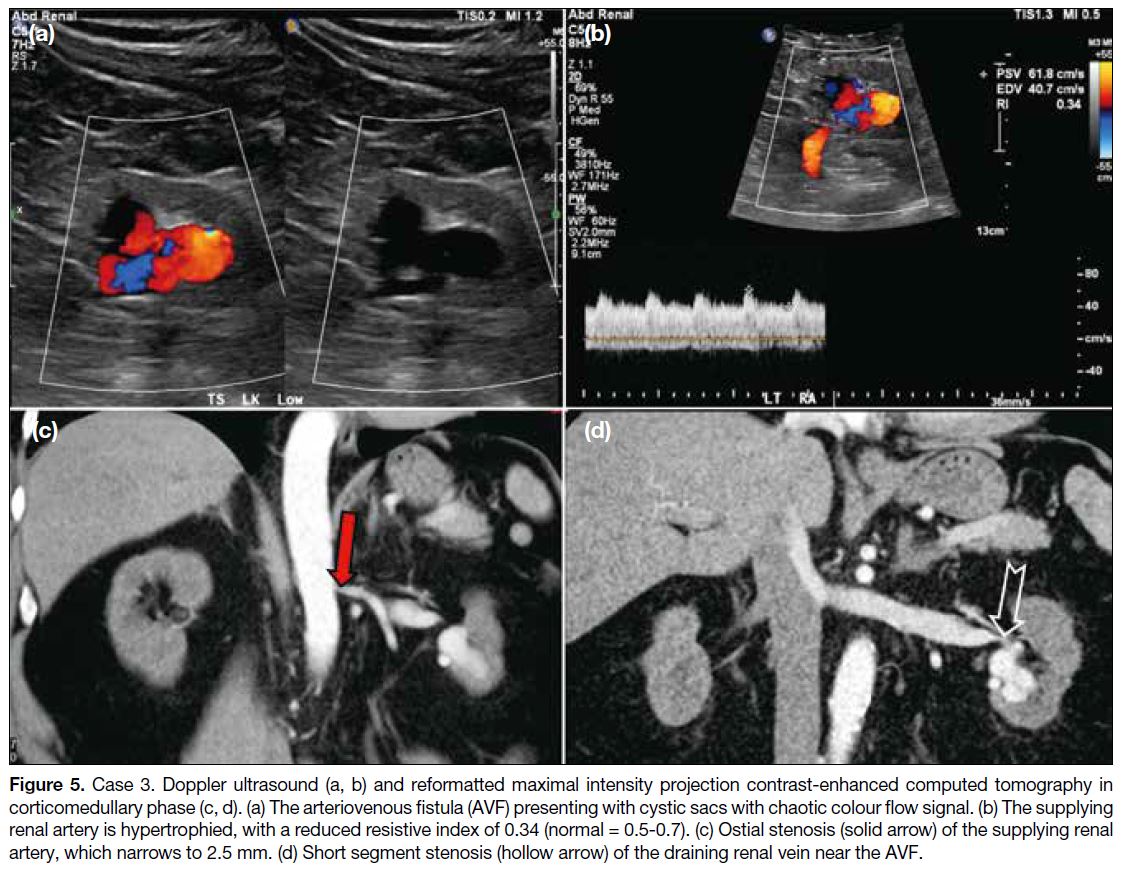
shown as a cystic area with moderate vascularity (Figure 5a and b) was incidentally detected on ultrasound and
was presumed biopsy-related. Intervention was deemed
indicated by nephrologists and urologists in view of
the higher bleeding risk in end-stage renal failure. CT
urogram confirmed an AVF centred at the lower pole of
the left kidney with aneurysmal changes. Double renal
arteries were seen. One of the renal arteries directly
supplied the AVF and showed ostial stenosis and
hypertrophy (7 mm) [Figure 5c]. The ostium measured
2.5 mm, limiting the option of sheaths and catheters.
A short segment tight stenosis was seen in the dilated
draining renal vein proximally near the renal hilum (Figure 5d), which minimised the migration of embolic agents.
Figure 5. Case 3. Doppler ultrasound (a, b) and reformatted maximal intensity projection contrast-enhanced computed tomography in
corticomedullary phase (c, d). (a) The arteriovenous fistula (AVF) presenting with cystic sacs with chaotic colour flow signal. (b) The supplying
renal artery is hypertrophied, with a reduced resistive index of 0.34 (normal = 0.5-0.7). (c) Ostial stenosis (solid arrow) of the supplying renal
artery, which narrows to 2.5 mm. (d) Short segment stenosis (hollow arrow) of the draining renal vein near the AVF.
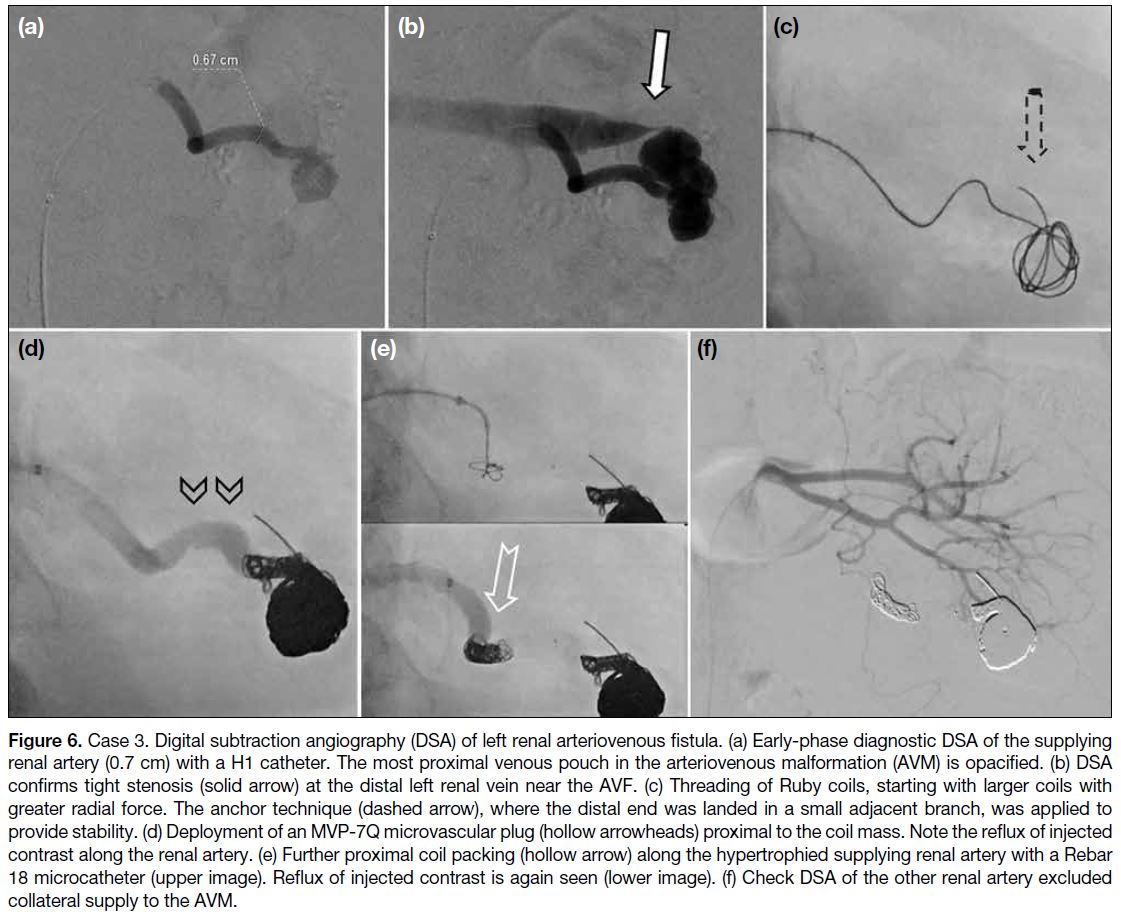
The supplying renal artery was cannulated with a 5-Fr
H1 catheter (Torcon NB Advantage Catheter; Cook
Medical, Bloomington [IN], US) [Figure 6a]. The most
proximal venous pouch was selectively cannulated
(Excelsior XT-27). Using the scaffold technique, the
venous pouch was packed with coils of varying lengths
and calibres (Ruby coils) [Figure 6c]. A microvascular
plug (MVP-7Q; Medtronic, Minneapolis [MN], US)
was launched at the supplying left renal artery [Figure 6d], followed by more proximal deployment of two
detachable coils [Figure 6e]. Check DSA confirmed
significant reduction in AVF vascularity and absence of
collateral supply from the other renal artery (Figure 6f).
Figure 6. Case 3. Digital subtraction angiography (DSA) of left renal arteriovenous fistula. (a) Early-phase diagnostic DSA of the supplying
renal artery (0.7 cm) with a H1 catheter. The most proximal venous pouch in the arteriovenous malformation (AVM) is opacified. (b) DSA
confirms tight stenosis (solid arrow) at the distal left renal vein near the AVF. (c) Threading of Ruby coils, starting with larger coils with
greater radial force. The anchor technique (dashed arrow), where the distal end was landed in a small adjacent branch, was applied to
provide stability. (d) Deployment of an MVP-7Q microvascular plug (hollow arrowheads) proximal to the coil mass. Note the reflux of injected
contrast along the renal artery. (e) Further proximal coil packing (hollow arrow) along the hypertrophied supplying renal artery with a Rebar 18 microcatheter (upper image). Reflux of injected contrast is again seen (lower image). (f) Check DSA of the other renal artery excluded collateral supply to the AVM.
DISCUSSION
To preserve renal function, endovascular treatment
has become the mainstay treatment of renal AVF in
the current literature. For non-traumatic AV shunts,
Marunos et al[5] proposed corresponding treatment
modalities based on three types of angioarchitecture.
Type I involves single or few arteries shunting to a
dilated single draining vein, while type II contains
multiple arterioles shunting to a single dilated draining
vein. Coils are recommended in these two types, while
vascular plugs can be considered in type I shunts. For
type III, where multiple connections exist between
arterioles and venules, particles and liquid embolic
agents are recommended. Proximal embolisation of the
arterial feeder with coils in type III shunts should be
avoided to prevent recruitment of collaterals. Traumatic shunts, which usually present with pseudoaneurysms, are
located peripherally and have similar angioarchitecture
to type I shunts. As well as coils, glue is a treatment
option. These endovascular treatment modalities are
considered effective and are commonly used in clinical
practice.
Detachable coils allow precise deployment and have low
risk of non-target embolisation in a high-flow setting.
Particles and liquid embolic agents are time-efficient in
type III profiles but carry risks of proximal and non-target
embolisation. The combination of distal coil anchor
and proximal vascular plug is gaining in popularity,
with reported success in recanalised[6] and giant AVF,[2]
although limited case numbers mean its superiority has
not been validated. Plugging is an efficient alternative
to coil mass, but a straight non-conical landing zone is required. The maximum sizes that the Amplatzer or the
MVP Micro Vascular Plug system offer may also limit
their application in enlarged feeders. For Amplatzer
vascular plugs, serial deployment may be considered
to achieve optimal flow control, especially for larger
plugs due to their larger pore size.[7] In our experience,
deployment of a single plug may be insufficient for flow
control. It is therefore our preference to perform distal
coil packing.
AVFs impose an elevated risk of distal non-target
embolisation due to their high-flow nature. To provide
coil stability, the double-catheter technique (Case 1)
or the side-branch anchor technique (Case 3) can be
performed. Flow modulation with occlusive balloons
applied proximal (Case 1) and distal to the fistula
also provides stability for the initial coil framework.[8] [9] The ‘pre-framing’ technique, which involves coiling
the microcatheter in the designated area prior to coil
deployment, has also been practised.[10] The rigidity
of mechanically detachable or larger-sized coils is
nonetheless technically difficult since the coils traverse
through the tortuous catheter framework. It also risks
catheter knotting and requires a side branch for the
microcatheter to anchor upon. Alternatively, the use of
covered or constrained stents for coil trapping has been
successful.[3]
CT or MR arteriography provides an excellent roadmap
for preprocedural planning and a crude estimation of the
post-embolisation residual functional kidney. In Case
3 for example, ostial stenosis limited catheter sizing
and subsequent choice of embolic agents. The venous
outflow should also be carefully studied. A grossly
dilated vein, as in Case 1, implies a high risk of distal
non-target embolisation. A venous occlusive balloon is
most reported to prevent distal embolisation. Suprarenal
inferior vena cava filters may also be considered but their application is limited in flow-induced mega cava, as in
Case 1. The use of an atrial septal defect occluder has
also been reported.[11] Embolisation of the venous outflow
tract is not commonly practised and not necessarily
indicated when feeder obliteration is achieved. It may be
considered when multifocal feeders are present, where
extensive embolisation would result in lowered nephron-sparing
capacity.
CONCLUSION
This case series is based on single-centre experience
with a small sample size. Some cases were excluded,
including those with difficult vascular anatomy and
concerns about compromising renal function.
Endovascular treatment of three selected cases of renal
AVF is illustrated. Various treatment modalities have
been proven successful and may be selected according
to the angioarchitecture. The combination of coil and
plug is gaining popularity. The high-flow nature of AVF
requires careful preprocedural planning and additional intra-procedural manoeuvres to minimise the risk
of embolic agent migration. Target coiling of larger
aneurysms also contributes to treatment success.
REFERENCES
1. Nagpal P, Bathla G, Saboo SS, Khandelwal A, Goyal A, Rybicki FJ,
et al. Giant idiopathic renal arteriovenous fistula managed by coils
and Amplatzer device: case report and literature review. World J
Clin Cases. 2016;4:364-8. Crossref
2. Yoneda S, Madono K, Tanigawa G, Fujita K, Yazawa K, Hosomi M, et al. Case of giant renal arteriovenous fistula in a long-term hemodialysis patient [in Japanese]. Hinyokika Kiyo.
2009;55:559-62.
3. Resnick S, Chiang A. Transcatheter embolization of a high-flow
renal arteriovenous fistula with use of a constrained wallstent to
prevent coil migration. J Vasc Interv Radiol. 2006;17:363-7. Crossref
4. Shie RF, Su TW, Hsu MY, Chu SY, Ko PJ. Transarterial
embolization of a large high-flow right renal arteriovenous fistula
using stents and an across-stent wire-trapping technique. J Vasc
Surg Cases Innov Tech. 2019;5:122-7. Crossref
5. Maruno M, Kiyosue H, Tanoue S, Hongo N, Matsumoto S,
Mori H, et al. Renal arteriovenous shunts: clinical features,
imaging appearance, and transcatheter embolization based on angioarchitecture. Radiographics. 2016;36:580-95. Crossref
6. Abdel-Aal AK, Elsabbagh A, Soliman H, Hamed M, Underwood
E, Saddekni S. Percutaneous embolization of a postnephrectomy
arteriovenous fistula with intervening pseudoaneurysm using the
Amplatzer Vascular Plug 2. Vasc Endovascular Surg. 2014;48:516-21. Crossref
7. Balasubramanian K, Keshava SN, Lenin A, Mukha R. Endovascular
management of a patient with massive renal arteriovenous fistula:
challenges and tricks. BMJ Case Rep. 2021;14:e236358. Crossref
8. Idowu O, Barodawala F, Nemeth A, Trerotola SO. Dual use of an
Amplatzer device in the transcatheter embolization of a large high-flow
renal arteriovenous fistula. J Vasc Interv Radiol. 2007;18:671-6. Crossref
9. Mansueto G, D’Onofrio M, Minniti S, Ferrara RM, Procacci C.
Therapeutic embolization of idiopathic renal arteriovenous fistula
using the “stop-flow” technique. J Endovasc Ther. 2001;8:210-5. Crossref
10. Sundarakumar DK, Kroma GM, Smith CM, Lopera JE, Suri R.
Embolization of a large high-flow renal arteriovenous fistula
using 035” and 018” detachable coils. Indian J Radiol Imaging.
2013;23:151-4. Crossref
11. Chen X, Zeng Q, Ye P, Miao H, Chen Y. Embolization of high-output
idiopathic renal arteriovenous fistula primarily using an
atrial septal defect occluder via venous access: a case report. BMC
Nephrol. 2019;20:15. Crossref