Magnetic Resonance Imaging–guided Biopsy of the Breast: A Ten-Year Experience
ORIGINAL ARTICLE
Magnetic Resonance Imaging–guided Biopsy of the Breast: A Ten-Year Experience
CCY Chan, EPY Fung, WP Cheung, KM Kwok, WS Mak, KM Wong, LW Lo, A Wong, D Fenn, AYH Leung
Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China
Correspondence: Dr CCY Chan, Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China. Email: chancherrycy@gmail.com
Submitted: 29 September 2023; Accepted: 27 August 2024.
Contributors: CCYC, EPYF, WPC and KMK designed the study. CCYC acquired the data. CCYC and EPYF analysed the data. CCYC drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, CCYC was not involved in the peer review process. Other authors disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: KC/KE-23-0152/ER-2). Informed patient consent was waived by the Board due to the retrospective nature of the research.
Abstract
Introduction
Magnetic resonance imaging (MRI) is an effective modality for high-risk patient screening, local
staging, and disease monitoring in breast malignancies. There is an increasing demand for MRI-guided biopsy of
lesions that are occult on mammography or ultrasound. This study summarises our 10-year experience with the
procedure. Technical challenges, as well as tips and tricks to achieve procedural success are discussed.
Methods
A total of 37 consecutive cases of MRI-guided vacuum-assisted breast biopsies performed at a single centre
between August 2012 and August 2023 were retrospectively reviewed. Targets were localised using 1.5-T MRI systems
with a dedicated breast coil and localisation device. Biopsies were performed using 10-gauge or 9-gauge needles.
Imaging characteristics, histopathological results, and subsequent management for all biopsied lesions were recorded.
Results
The mean age of patients was 51.6 years. Technical success was achieved in 35 out of 37 cases (94.6%). In
27 cases (77.1%), the biopsied breast was placed in the ipsilateral coil, and in eight cases (22.9%) it was placed in
the contralateral coil for optimal imaging and biopsy access. Between 8 and 24 cuttings were taken for each target.
Three cases (8.6%) developed biopsy site haematomas. Of the 35 successfully biopsied lesions, 11 (31.4%) were
malignant. Among the malignant lesions, six (54.5%) presented as non-mass enhancement and five (45.5%) as mass
enhancement. Four lesions (36.4%) showed restricted diffusion, while seven (63.6%) did not.
Conclusion
MRI-guided vacuum-assisted biopsy of the breast is a safe and effective procedure in the hands of experienced interventionists. It is essential for the diagnosis and management of breast lesions occult on conventional imaging.
Key Words: Breast neoplasms; Fibroadenoma; Image-guided biopsy; Magnetic resonance imaging; Mammography
中文摘要
磁力共振成像引導乳腺活檢檢查:十年經驗分享
陳卓忻、馮寶恩、張偉彬、郭勁明、麥詠詩、黃嘉敏、羅麗雲、黃皓澧、范德信、梁燕霞
引言
磁力共振成像是乳腺惡性腫瘤高風險患者篩檢、局部分期和病情監測的有效方法。對於乳房造影掃瞄或超聲波檢查中難以發現的病變,磁力共振成像引導下活檢的需求日益增長。本研究總結了我們十年來在該技術上的經驗,並探討其中的技術挑戰以及確保活檢成功的技巧和竅門。
方法
我們對2012年8月至2023年8月期間在同一中心進行的連續37例磁力共振成像引導下真空輔助乳腺活檢病例進行回顧性分析。我們使用配備專用乳腺線圈和定位裝置的1.5 T磁力共振成像系統進行標靶區定位。活檢採用10號或9號穿刺針。我們記錄了所有活檢病變的影像學特徵、組織病理學結果及後續處理。
結果
患者平均年齡為51.6歲。 37例患者中35例(94.6%)技術成功。活檢時27例(77.1%)乳腺置於同側線圈內,8例(22.9%)乳腺置於對側線圈內,以獲得最佳影像及活檢入路。每個目標活檢8至24個乳腺組織。三例(8.6%)出現活檢部位血腫。在35個成功活檢的病灶中,11例(31.4%)為惡性病灶。在惡性病灶中,6例(54.5%)表現為無腫塊強化,5例(45.5%)表現為腫塊強化。四例(36.4%)病灶顯示彌散受限,7例(63.6%)未顯示彌散受限。
結論
磁力共振成像引導下乳腺真空輔助活檢在經驗豐富的介入醫生操作下是一種安全有效的操作,對於常規影像學檢查無法確診的乳腺病變的診斷和治療至關重要。
INTRODUCTION
Magnetic resonance imaging (MRI) of the breast is an
effective modality for screening high-risk patients,
local staging and monitoring breast malignancies that
are occult on radiography and sonography. This article
summarises our centre’s experience in MRI-guided
breast biopsy over the past 10 years, with the aim of
reviewing the fundamentals of the procedure and
emphasising tips and tricks for technically challenging
cases.
MAGNETIC RESONANCE IMAGING–GUIDED BREAST BIOPSY
Indications and Contraindications
MRI of the breast is performed for indications as
categorised by the European Society of Breast Cancer
Specialists working group, including screening for high-risk
patients such as those with a strong family history
of breast malignancies or known genetic mutations, e.g.,
BRCA1 and BRCA2; characterisation of inconclusive
findings on mammography or ultrasound; assessment
for unknown primary breast cancer; preoperative local
staging and surgical planning in patients with biopsy-proven
breast malignancy (particularly those considered
for breast conserving therapy); and disease monitoring
in known breast malignancies, e.g., evaluating treatment
response to neoadjuvant chemotherapy.[1] When a
suspicious lesion occult on conventional breast imaging
(i.e., mammography and ultrasound) is detected on
MRI, an MRI-guided biopsy should be performed if a
targeted second-look ultrasound is unyielding according
to the American College of Radiology (ACR)[2] and the
European Society of Breast Imaging[3] recommendations.
Absolute contraindications to MRI-guided breast
biopsy are the same as for any MRI scan, including the
presence of MRI-incompatible metallic or magnetic
implants, claustrophobia, contrast allergy, or severe
renal impairment.[4] Relative contraindications include
thrombocytopenia and coagulopathies.[4]
Magnetic Resonance Imaging Protocol
At our centre, MRI-guided breast biopsy is performed
using one of two 1.5-T MRI systems (Achieva XR; Philips
Healthcare, Best, the Netherlands, and MAGNETOM
Sola; Siemens Healthcare, Erlangen, Germany) with
phased-array dedicated breast coils containing four or seven channels, respectively. The MRI protocol for
biopsy differs from the usual diagnostic protocol by using
breast coils with fewer channels and prioritising rapid
image acquisition with sequences optimised for target
localisation.[5] Our protocol consists of T1-weighted pre- and
post-contrast sequences acquired in 1-mm section
thickness. Gadoterate meglumine is the gadolinium-based
contrast medium of choice, administered at the
recommended dose of 0.2 mL/kg (or 0.1 mmol/kg) at a
rate of 2 mL/s via a pump injector. Dynamic T1-weighted
three-dimensional fat-saturated images are acquired,
with the first set of post-contrast images obtained 1.5
to 2 minutes after injection, followed by a second set at
a 25-second interval, and then at 1-minute intervals up
to 4 minutes, as recommended by the ACR guidelines.[2]
Subtraction of the unenhanced images is performed.
Patient Positioning
Optimal patient positioning is crucial for procedural
success. The patient is positioned prone with cushion
support for the head and neck, and a headset for noise
cancellation. Arms are positioned overhead with
padding at the sternum, abdomen, and legs for comfort
and stability. The targeted breast is placed hanging freely
and as deeply and centrally as possible in the dedicated
breast coil with the nipple pointing directly downwards.[6]
The operator should ensure there are no breast folds
resulting from compression at the edge of the coil, as this
leads to uneven fat saturation on MRI.[7]
Lesion Localisation
Once the patient is optimally positioned, breast
compression is performed using a grid paddle. Pre-contrast
MRI is conducted to verify breast placement
to ensure the target falls within the multichannel
localisation grid.[6] A fiducial marker, either an MRI-visible
fish oil capsule or a small plastic marker is affixed
to skin of the breast within the grid square, close to, but
not directly over, the anticipated location of the target.
Contrast is administered and MRI images are acquired
as per protocol. Post-processing subtraction images are
generated to localise the target.
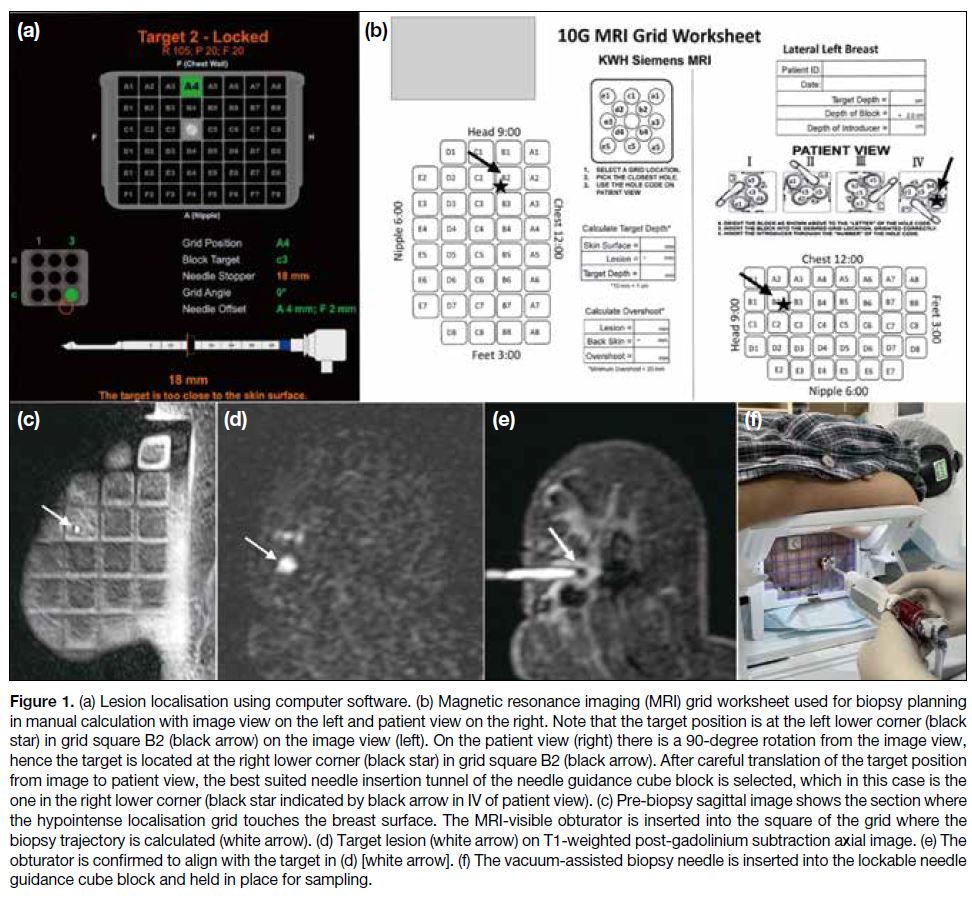
Following localisation, the biopsy tract is planned either
manually or using the computer-assisted localisation
software[4] [6] (syngo MR XA 51; Siemens, Erlangen,
Germany) [Figure 1a]. In the manual approach, an
MRI grid worksheet (Figure 1b) with two sets of views,
namely, the patient view and the MRI view, is used to
select the localisation grid channel and needle tunnel
based on calculation of lesion coordinates. The patient view represents a 90° anti-clockwise rotation of the
sagittal MRI image, as in reality the patient lies prone.
The image section where the hypointense localisation
grid contacts the skin surface is taken as the first section
(Figure 1c). The number of sections from this point to
the target is multiplied by section thickness to determine
lesion depth. The thickness of the needle guidance cube
block (2 cm) is then added for calculation of overall
needle insertion depth. Distances between the fiducial
marker and the target along the horizontal and vertical
axes are also measured. It is important to verify correct
laterality (i.e., left or right breast) and approach (i.e.,
lateral or medial) when selecting the worksheet as each
is different; and to carefully translate the target from the
MRI view to the patient view by turning anti-clockwise
of the clock face or direction by 90° on the worksheet,
as any mistake in these steps will result in inaccurate
targeting. Alternatively, the computer localisation
software automatically calculates lesion coordinates
and indicates the specific square of the multichannel
localisation grid, the tunnel within the needle guide
cube block, and the required needle insertion depth for
accurate targeting.[6]
Figure 1. (a) Lesion localisation using computer software. (b) Magnetic resonance imaging (MRI) grid worksheet used for biopsy planning
in manual calculation with image view on the left and patient view on the right. Note that the target position is at the left lower corner (black
star) in grid square B2 (black arrow) on the image view (left). On the patient view (right) there is a 90-degree rotation from the image view,
hence the target is located at the right lower corner (black star) in grid square B2 (black arrow). After careful translation of the target position
from image to patient view, the best suited needle insertion tunnel of the needle guidance cube block is selected, which in this case is the
one in the right lower corner (black star indicated by black arrow in IV of patient view). (c) Pre-biopsy sagittal image shows the section where
the hypointense localisation grid touches the breast surface. The MRI-visible obturator is inserted into the square of the grid where the
biopsy trajectory is calculated (white arrow). (d) Target lesion (white arrow) on T1-weighted post-gadolinium subtraction axial image. (e) The
obturator is confirmed to align with the target in (d) [white arrow]. (f) The vacuum-assisted biopsy needle is inserted into the lockable needle
guidance cube block and held in place for sampling.
Biopsy
Following injection of local anaesthesia and skin incision
at the expected needle position based on calculated
lesion coordinates, a small lockable needle guidance
cube block is inserted into the localisation grid channel
(i.e., one of the many channels/boxes from the square
grid; A1 to F8 in Figure 1a) over the skin incision and
secured. The numerically labelled plastic introducer
sheath, with its depth stop set to the calculated insertion
depth, together with the inner non-ferrous metallic
trocar, is inserted into one of the tunnels of the needle
guidance cube block, which is best positioned over the
skin incision, and the coaxial system is advanced to
the calculated lesion depth. The metallic trocar is then
replaced with an EnCor MRI-visible obturator (BD Inc,
Franklin Lakes [NJ], US) and MRI images are acquired
to confirm the alignment of the obturator with the target
(Figure 1d and e). The obturator is then removed and the
vacuum-assisted biopsy needle is inserted to the same
calculated depth. The aperture of the biopsy needle is
oriented to face in the direction of the lesion relative to the
selected needle tunnel, a technique known as ‘directional
sampling’.[7] Vacuum-assisted biopsy is then performed
(Figure 1f) with the desired number of cuttings and the
needle is removed. A post-biopsy MRI scan is acquired
to confirm the correct site and adequate sampling of the
target.
After Biopsy
After sampling, an MRI-compatible biopsy marker is
inserted via the biopsy tract through the introducer sheath
and deployed at the biopsy site. Acquisition of post-marker
insertion images is optional and not routinely
performed, as haematoma or gas artefacts often obscures
the marker.[8] All needles are removed and haemostasis
is achieved by manual compression of the breast for at
least 15 minutes.
Complications
Bleeding and haematoma formation at biopsy site are
the most common complications.[9] Other complications
include infection and muscle injury (i.e., injury to the pectoralis muscles). Rare complications include
pneumothorax and injury to mediastinal structures,
which only occur when biopsy is performed using a
freehand approach without a localisation grid.[9]
METHODS
Thirty-seven consecutive cases of MRI-guided
vacuum-assisted breast biopsies performed between
August 2012 and August 2023 in a single centre were
retrospectively reviewed. Target lesions were localised
using the Philips or Siemens 1.5-T MRI system
with dedicated breast coils and an Invivo (Philips,
Amsterdam, Netherlands) or Breast BI 7 Coil (Siemens,
Höchberg, Germany) localisation device. Biopsies were performed using 10-gauge EnCor or 9-gauge
Suros (Hologic, Marlborough [MA], US) needles.
Imaging characteristics including size, morphology, and
enhancement pattern were recorded. Histopathology
of all biopsied lesions was obtained with subsequent
management documented.
RESULTS
The mean age of patients was 51.6 years (range, 33-76). Technical success was achieved in 35 out of 37
cases (94.6%). In three cases, the original target was
not visualised on pre-procedural MRI, resulting in
cancellation of the procedure in two cases, while a
nearby target was selected in the remaining case.
In 27 cases (77.1%), the lateral approach was adopted
and the biopsied breast was placed in the ipsilateral coil.
In eight cases (22.9%), the medial approach was used,
and the breast was placed in the contralateral coil. A
total of 34 lesions were biopsied using a 10-gauge EnCor
needle and one lesion was biopsied using a 9-gauge Suros
needle. Between 8 and 24 cuttings were taken for each
target, with an average of 13 cuttings made. Three cases
(8.6%) were complicated by biopsy-site haematomas:
two were managed by prolonged manual compression
for more than 30 minutes and one required aspiration of
the haematoma using the vacuum-assisted biopsy device
followed by manual compression. Haemostasis was
successfully achieved in all three cases.
Table 1 shows the histology of the lesions and Table 2 shows the malignant diagnoses. Of the 11 malignant
lesions, one case (9.1%) yielded a false-negative
result from MRI-guided biopsy and proceeded to
surgical excision after consensus was reached at
the multidisciplinary meeting due to clinical and
radiological-histopathological discordance. Histology
from the surgical specimen revealed invasive ductal
carcinoma (Table 2).
Table 1. Histopathological results of biopsied lesions (n = 35)
Table 2. Histopathological results of the biopsied lesions positive
for malignancy (n = 11)
Six malignant lesions (54.5%) presented as non-mass
enhancement and five as mass enhancement (45.5%).
The size of the malignant lesions ranged from 0.5 cm
to 4.3 cm. Four lesions (36.3%) showed restricted
diffusion, while seven (63.6%) did not. Nine malignant
lesions (81.8%) exhibited a type II enhancement curve
and two (18.2%) demonstrated a type III enhancement
curve. Ten malignant lesions (90.9%) were classified
as BI-RADS (Breast Imaging Reporting and Data
System)[10] category 4 and the remaining case (9.1%) as
category 5. All but one patient underwent surgery with either mastectomy or breast conserving therapy; the
remaining patient declined surgery and remained under
regular clinical and radiological follow-up.
DISCUSSION
MRI-guided vacuum-assisted biopsy of the breast is a
technically demanding procedure requiring specialised
equipment and a skilled, well-trained team with
appropriate experience. Various factors contribute to
procedural success. First, patient safety in the MRI
suite should be ensured for a smooth procedure. Any
equipment entering the suite should be carefully
examined for MRI compatibility.[2] A trolley is usually
prepared for the transport of equipment in and out of
the suite between image acquisition and biopsy, and
all metallic devices must be removed during image
acquisition. Second, efficiency is essential for successful
biopsy owing to limited timeframe between lesion
enhancement and contrast washout, while progressive
background parenchymal enhancement further obscures
targets.[7] It is also important to note that patients are often
placed in an uncomfortable position and may move during
prolonged procedures, resulting in lesion motion and
therefore sampling failure.[7] Meticulous preprocedural
planning with review of the diagnostic MRI, education
and communication with the patient prior to biopsy to
reduce anxiety and manage expectation, particularly
regarding the importance to stay still throughout the
procedure, and efficient execution of each biopsy step
is therefore crucial to achieve procedural success. The following are tips and tricks accumulated over the years
to address challenging cases.
Patient selection
Compression Technique
Controlled compression of the breast with moderate
pressure is performed with a grid paddle and should
be adequate for immobilisation with the breast just
taut. Inadequate compression increases the risk of
mistargeting due to breast and lesion motion throughout
the procedure, while excessive compression increases
patient discomfort and may impede blood flow to the
breast, resulting in reduced or non-enhancement of the
target leading to localisation failure.[9]
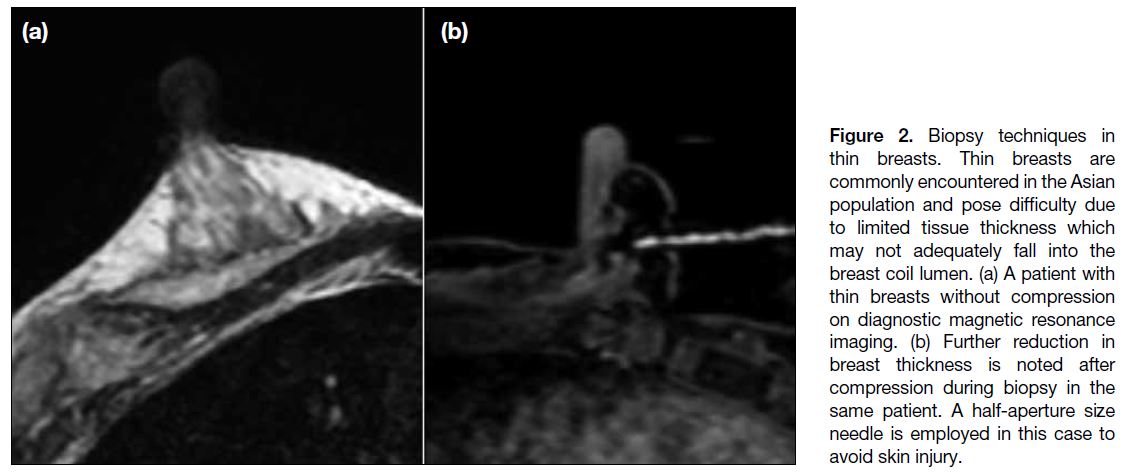
Thin Breasts
Thin breasts (Figure 2a) present unique challenges.
Target lesions may not fall adequately into the breast coil
to allow needle access. Optimising patient positioning
can markedly improve procedural success, whereby chest
pads can be removed from the coil or replaced by thinner
pads, and the patient can be tilted into an oblique position,
allowing the breast to drop further into the coil lumen.[11]
After compression, breast thickness is further reduced
(Figure 2b) and may be inadequate to accommodate the
standard biopsy needle, therefore compression may be
reduced to increase tissue thickness to allow biopsy.[12]
Local anaesthesia can also be injected either anterior or
posterior to the target to increase distance between skin
and the target to accommodate the biopsy needle. A
blunt tip needle, half-aperture size needle or petit needle (e.g., 13-gauge) can be employed to minimise chest wall
injury or contralateral skin penetration risks.[11]
Figure 2. Biopsy techniques in
thin breasts. Thin breasts are
commonly encountered in the Asian
population and pose difficulty due
to limited tissue thickness which
may not adequately fall into the
breast coil lumen. (a) A patient with
thin breasts without compression
on diagnostic magnetic resonance
imaging. (b) Further reduction in
breast thickness is noted after
compression during biopsy in the
same patient. A half-aperture size
needle is employed in this case to
avoid skin injury.
Lesion Located at The Cross of Localisation Grid
Squares
Occasionally, the target may fall onto the intersection
of localisation grid squares after injection of local
anaesthesia as shown in Figure 1a (red circle). In such
cases, directional sampling and appropriate manoeuvring
will significantly improve procedural success.[7] The
needle guidance cube block is inserted into the A4
square and the biopsy needle is inserted into the tunnel
of the cube block indicated by computer software (tunnel
c3; highlighted in green), then directional sampling is
performed with the aperture of the biopsy needle facing
the 7 to 8 o’clock position aiming at the target, while
manual pressure is applied by the operator’s finger
through another grid square (B4 in this case) in attempts
to push the breast tissue and hence the target towards the
direction of the biopsy needle to aid sampling (Figure 1a). Niketa et al[11] also described a biopsy technique
with two diagonally placed entry sites in adjacent holes
paired with directional sampling technique to improve
sampling success in such cases.
Location of Lesions in the Breast
Anterior Lesions
For anterior lesions, with large breasts, they may touch the table and distort the breast, rendering localisation
difficult. Padding can be added to raise the body from
the coil so that the target is more easily reached.
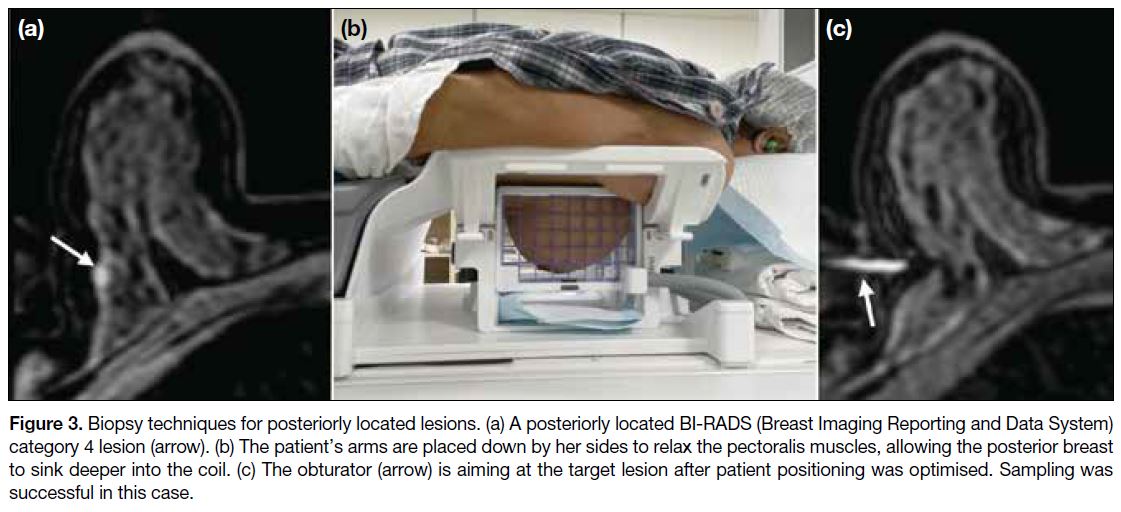
Posterior Lesions
For lesions close to the chest wall (Figure 3a), removing
coil cushion covers brings the chest closer to the coil
aperture. The arms can be placed down by the sides of
the patient instead of above the head, as this relaxes the
pectoralis muscles and allows the breast to sink deeper
into the coil (Figure 3b). However, if the target is too
close to the pectoralis muscles, the arms should be placed
above the head to help retract the muscle away from
the coil lumen to avoid muscle injury, which can cause
excessive pain and haemorrhage. Tilting the patient into
an oblique position may also help the posterior parts of
the breast sink further (Figure 3c). On rare occasions,
the target may lie posterior to the localisation grid even
after these manoeuvres. Performing the biopsy posterior
to the grid or by freehand needle insertion without
breast compression and localisation grids has been
described.[11] The importance of maintaining stability of
the needle position in these scenarios is emphasised, as
the absence of support from the localisation grid and/or
immobilisation of the breast from compression increases
the difficulty of targeting.
Figure 3. Biopsy techniques for posteriorly located lesions. (a) A posteriorly located BI-RADS (Breast Imaging Reporting and Data System)
category 4 lesion (arrow). (b) The patient’s arms are placed down by her sides to relax the pectoralis muscles, allowing the posterior breast
to sink deeper into the coil. (c) The obturator (arrow) is aiming at the target lesion after patient positioning was optimised. Sampling was
successful in this case.
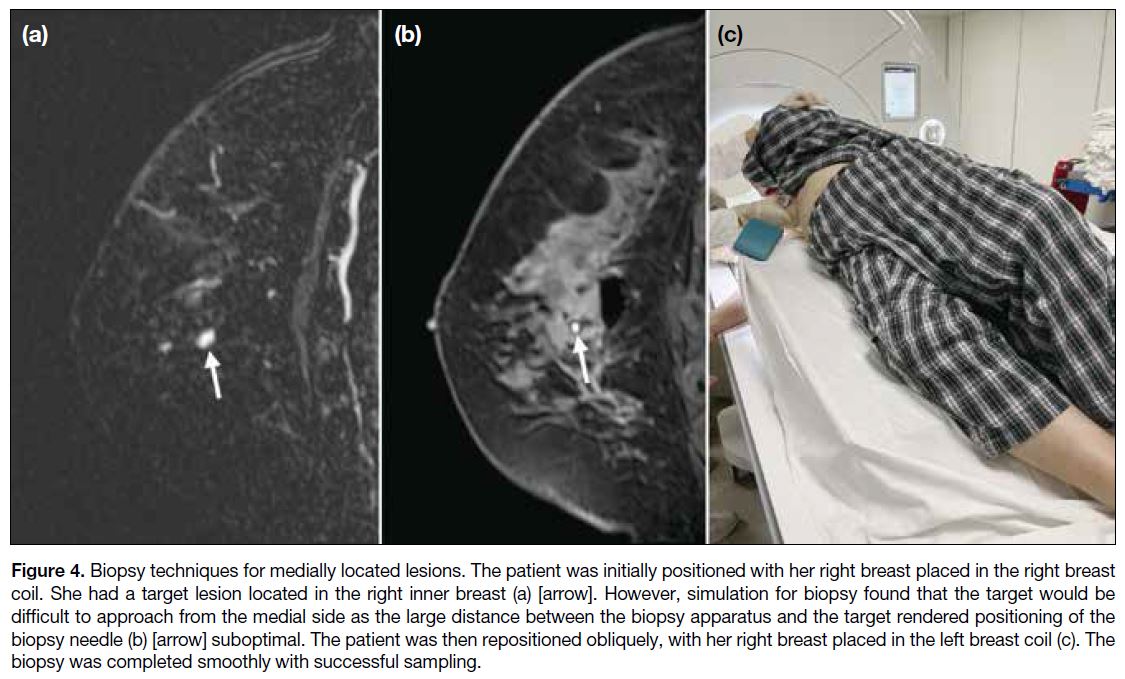
Medial Lesions
It is difficult to target medial lesions from the medial
side due to the increased distance between the biopsy
apparatus and the breast when the breast is placed in
an ipsilateral coil. The design of the breast coil, with a
downward slant from the lateral bar to the sternal bar,
also aggravates the difficulty of accessing posteromedial
lesions, as the further the biopsy needle travels, the more anteriorly (towards the nipple) the needle tip will be
directed due to this angulation.[6] It is thus often helpful
to place the targeted breast in the contralateral breast coil
(i.e., the right breast in the left breast coil [Figure 4]),
which shortens the distance between the biopsy apparatus
and the target and reduces the downward angulation the
biopsy needle must overcome. This is a less comfortable
position for the patient due to the tilting, making it harder
to stay still. It is therefore vital for operators to optimise
patient comfort before commencement of biopsy to
minimise lesion motion. Another limitation to this
manoeuvre is that obese patients may not be able to fit
through the bore of the MRI.[6]
Figure 4. Biopsy techniques for medially located lesions. The patient was initially positioned with her right breast placed in the right breast
coil. She had a target lesion located in the right inner breast (a) [arrow]. However, simulation for biopsy found that the target would be
difficult to approach from the medial side as the large distance between the biopsy apparatus and the target rendered positioning of the
biopsy needle (b) [arrow] suboptimal. The patient was then repositioned obliquely, with her right breast placed in the left breast coil (c). The
biopsy was completed smoothly with successful sampling.
Superficial Lesions
Injury to the skin is the primary concern in these lesions.
If the needle aperture is not completely embedded
within the breast during biopsy, air leakage and loss of
vacuum effect may ensue, which further lower the rate of
successful sampling.[11] This can be tackled by generous
injection of local anaesthetic proximal to the target
to increase tissue depth to accommodate the biopsy
needle.[11] The biopsy needle can also be inserted a few
millimetres beyond the target so that the target falls into
the proximal part of the needle aperture. Alternatively,
smaller-aperture needles may be employed.
Periareolar Lesions
Biopsy around the nipple-areolar complex carries
increased risks of haemorrhage and pain as it is a highly
vascularised and innervated structure. It also raises cosmetic concerns. In these cases, the nipple-areolar
complex can be manually rolled away from the expected
site of skin incision and biopsy needle entry to avoid
injury to the complex.[11]
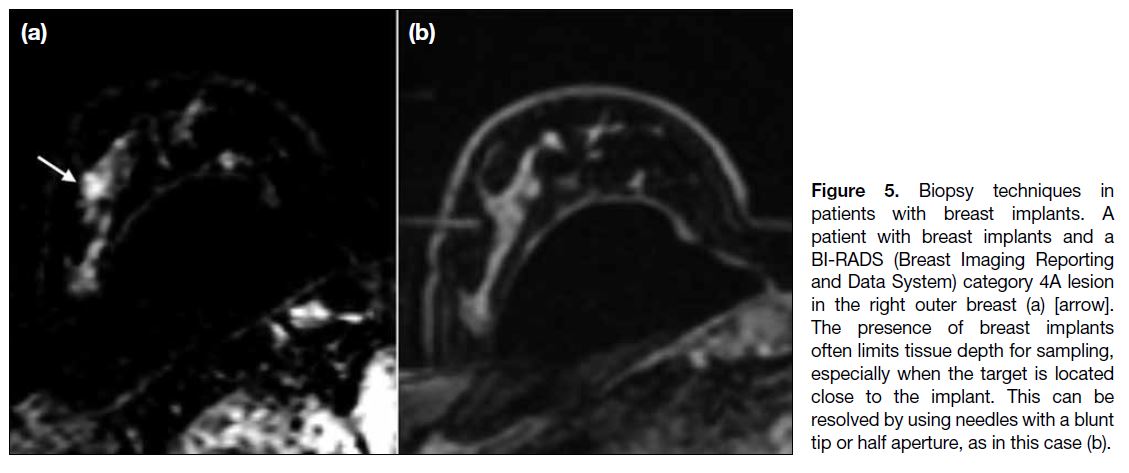
Breast Implants
Breast implants are increasingly common, and their
presence renders biopsy difficult. The operator must exercise extra caution not to puncture the capsule,
which may result in implant rupture. Adequacy of breast
parenchymal thickness should be assessed according to
the expected biopsy trajectory and if there is inadequate
tissue depth, half-aperture needles can be employed.[7]
If the target is located too close to the implant, blunt-tip
needles may minimise the risk of implant puncture
(Figure 5), or alternatively, fine needle aspiration can be performed instead.[12] Injection of local anaesthetic
between the target and the implant also allows tissue
dissection and increases the distance between the two,
providing more room for tissue sampling.
Figure 5. Biopsy techniques in
patients with breast implants. A
patient with breast implants and a
BI-RADS (Breast Imaging Reporting
and Data System) category 4A lesion
in the right outer breast (a) [arrow].
The presence of breast implants
often limits tissue depth for sampling,
especially when the target is located
close to the implant. This can be
resolved by using needles with a blunt
tip or half aperture, as in this case (b).
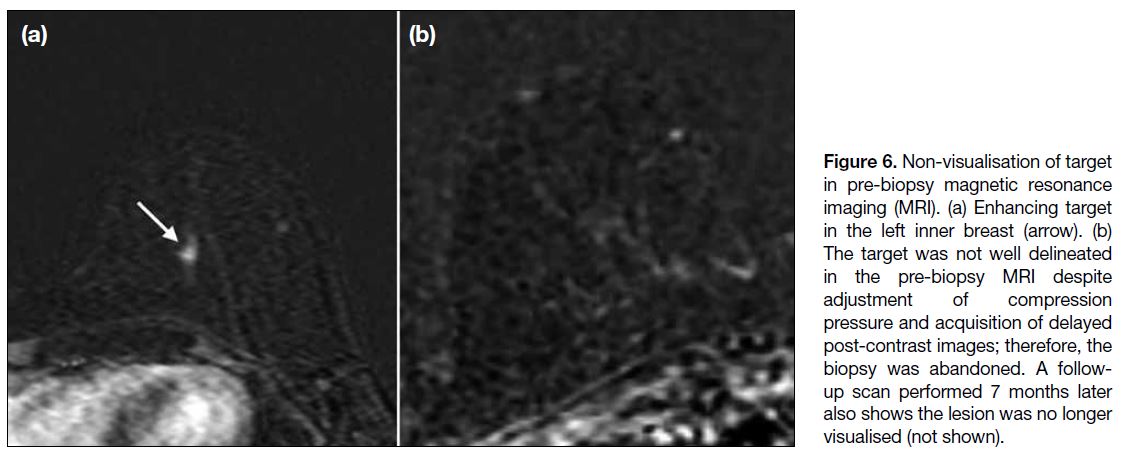
Non-visualisation of the Target in Pre-biopsy
Magnetic Resonance Imaging
Non-visualisation of the target (Figure 6) in preprocedural
MRI has been reported in approximately 8% to 13% of
cases.[13] Several factors should be taken into consideration
before abandoning biopsy. The diagnostic MRI should
be carefully reviewed to identify the sequences in
which the target is best visualised. For instance, the
lesion may be T2-hyperintense or shows restricted
diffusion on diffusion-weighted images (Figure 7), and
if it is not well delineated in the standard pre-biopsy
MRI sequences, these additional sequences should be
performed. This is also helpful when other non-target
lesions are conspicuous in these sequences and can be
identified as landmarks. Sometimes, the lesion may not
be identified due to inherent differences between the
breast coils used in diagnostic and pre-biopsy protocols,
as the latter includes a smaller number of channels,
which may lower the image quality. It is also important
to bear in mind that the breast is compressed using a grid
paddle in preprocedural MRI, whereas no compression
is applied during diagnostic MRI. Enhancement
dynamics of the target may therefore differ as blood
inflow may be impeded by compression of the breast,
preventing lesion enhancement and resulting in a false-negative
scan.[7] In such cases, the operator should verify
that compression pressure is not excessive and reduce
it if necessary. Another tip is to prolong post-contract image acquisition, e.g., at 1-minute intervals for up to 5
minutes, as lesion enhancement may be delayed due to
compression of the breast.[6] Non-visualisation can persist
after these manoeuvres due to several factors, including
fluctuation in background parenchymal enhancement
related to hormonal cycles and transient infective or
inflammatory process.[7] In such events, the biopsy should
be cancelled. However, non-visualisation of the target
during biopsy does not preclude malignancy, which has
been found in approximately 3.5% of such cases upon
follow-up imaging.[14] It is therefore prudent to perform
a follow-up MRI within 6 months upon cancellation of
biopsy according to ACR recommendations.[2]
Figure 6. Non-visualisation of target
in pre-biopsy magnetic resonance
imaging (MRI). (a) Enhancing target
in the left inner breast (arrow). (b)
The target was not well delineated
in the pre-biopsy MRI despite
adjustment of compression
pressure and acquisition of delayed
post-contrast images; therefore, the
biopsy was abandoned. A follow-up
scan performed 7 months later
also shows the lesion was no longer
visualised (not shown).
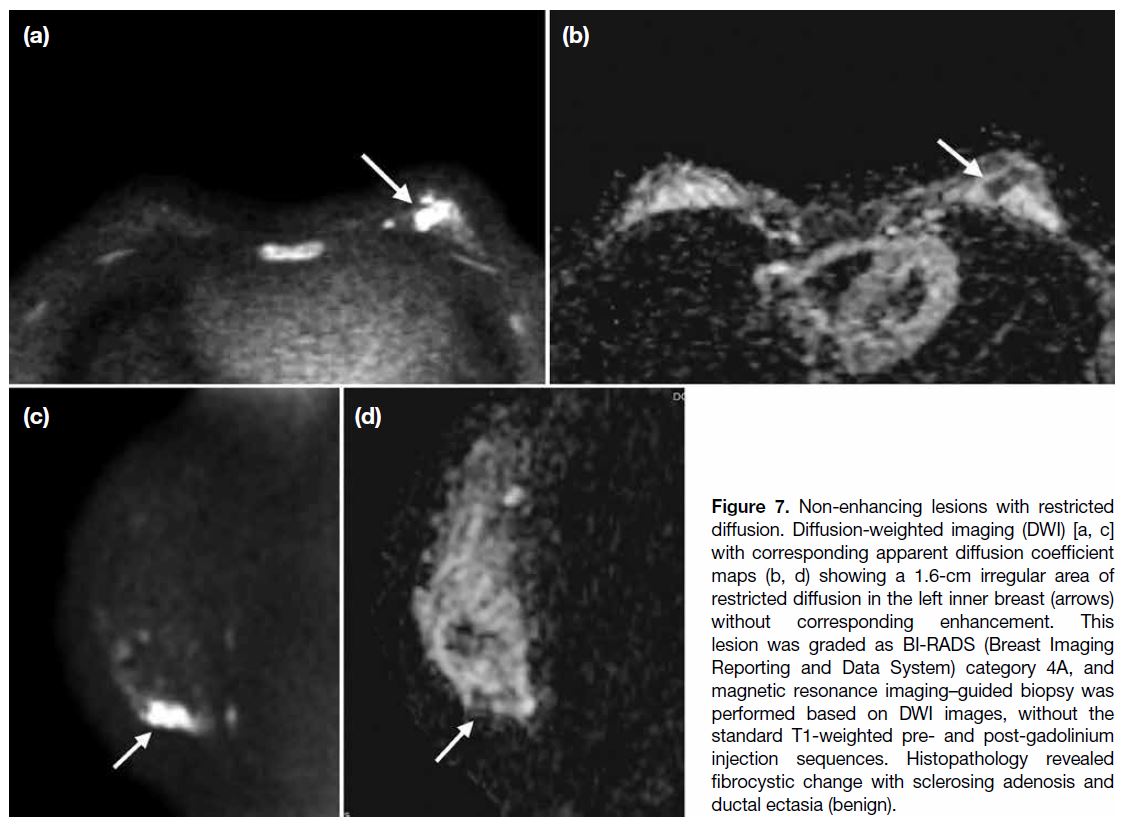
Figure 7. Non-enhancing lesions with restricted
diffusion. Diffusion-weighted imaging (DWI) [a, c]
with corresponding apparent diffusion coefficient
maps (b, d) showing a 1.6-cm irregular area of
restricted diffusion in the left inner breast (arrows)
without corresponding enhancement. This
lesion was graded as BI-RADS (Breast Imaging
Reporting and Data System) category 4A, and
magnetic resonance imaging–guided biopsy was
performed based on DWI images, without the
standard T1-weighted pre- and post-gadolinium
injection sequences. Histopathology revealed
fibrocystic change with sclerosing adenosis and
ductal ectasia (benign).
Postprocedural Haematoma
Manual compression is applied to the biopsied breast
for haemostasis for at least 15 minutes. A pressure
dressing or tight breast wraps can be used to facilitate
further compression afterwards. A sizeable biopsy site
haematoma may sometimes be seen on post-biopsy MRI.
In such cases, the vacuum-assisted biopsy needle can be
re-inserted through the co-axial system and switched to
aspiration mode for evacuation of the haematoma before
deployment of the biopsy marker (Figure 8). This often
reduces pain as well as minimises the risk of marker
displacement. In cases of uncontrolled bleeding with
suspected arterial injury, thrombin injection into the
biopsy cavity may be helpful for haemostasis control.[11]
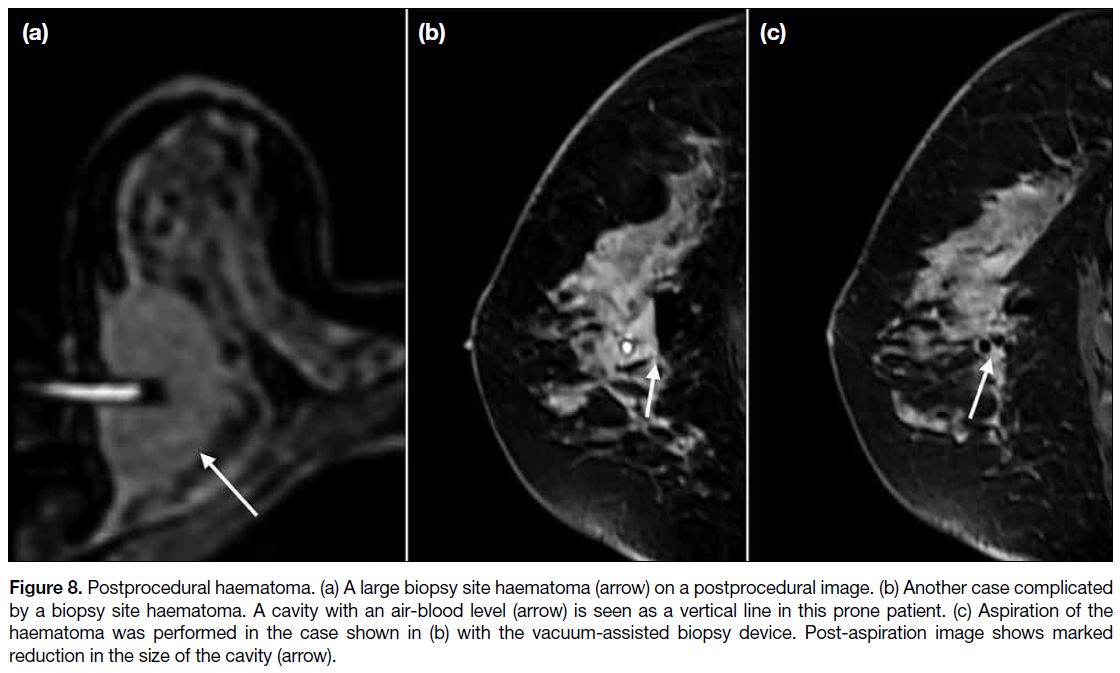
Figure 8. Postprocedural haematoma. (a) A large biopsy site haematoma (arrow) on a postprocedural image. (b) Another case complicated
by a biopsy site haematoma. A cavity with an air-blood level (arrow) is seen as a vertical line in this prone patient. (c) Aspiration of the
haematoma was performed in the case shown in (b) with the vacuum-assisted biopsy device. Post-aspiration image shows marked
reduction in the size of the cavity (arrow).
Radiological-Histopathological Discordance
Unlike ultrasound-guided biopsy where there is real-time visualisation of the biopsy trajectory and lesion,
or in stereotactic- or tomosynthesis-guided core biopsy where specimen radiographs confirms the presence of
calcifications, there is no direct method to assess targeting
accuracy in MRI-guided vacuum-assisted biopsy.
Radiological-histopathological concordance is therefore
of utmost importance to avoid missing any malignancies
in cases of suspicious imaging findings with negative
biopsy results.[15] [16] It is the operator’s responsibility to
review the histology results and report any discordance
to the surgical team, for which the next appropriate step
of management entails a repeated or excisional biopsy.
CONCLUSION
MRI has become an indispensable component of breast
imaging due to its high sensitivity in lesion detection.
However, its limited specificity, with significant overlap
of MRI characteristics between malignant and benign
lesions, highlights the importance of radiological-histopathological
correlation. It is therefore vital for
breast radiologists to understand the fundamentals
of MRI-guided vacuum-assisted biopsy in the face of its growing demands to achieve technical success
and guide the management of breast lesions occult on
mammography and ultrasound. MRI-guided vacuum-assisted
biopsy of the breast is a safe, feasible, and
effective procedure with high diagnostic yield in the
hands of experienced interventionists.
REFERENCES
1. Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;46:1296-316. Crossref
2. American College of Radiology. ACR Practice Parameter for
the Performance of Magnetic Resonance; Image-guided Breast
Interventional Procedures. Reston, VA: American College of
Radiology; 2016.
3. Mann RM, Balleyguier C, Baltzer PA, Bick U, Colin C, Cornford E,
et al. Breast MRI: EUSOBI recommendations for women’s
information. Eur Radiol. 2015;25:3669-78. Crossref
4. Papalouka V, Kilburn-Toppin F, Gaskarth M, Gilbert F. MRI-guided breast biopsy: a review of technique, indications, and radiological-pathological correlations. Clin Radiol. 2018;73:908.e17-25. Crossref
5. McGrath AL, Price ER, Eby PR, Rahbar H. MRI-guided breast interventions. J Magn Reson Imaging. 2017;46:631-45. Crossref
6. Price ER. Magnetic resonance imaging–guided biopsy of the breast: fundamentals and finer points. Magn Reson Imaging Clin N Am. 2013;21:571-81. Crossref
7. Santiago L, Candelaria RP, Huang ML. MR imaging–guided breast
interventions: indications, key principles, and imaging-pathology
correlation. Magn Reason Imaging Clin N Am. 2018;26:235-46. Crossref
8. Liberman L, Bracero N, Morris E, Thornton C, Dershaw DD.
MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical
experience. AJR Am J Roentgenol. 2005;185:183-93. Crossref
9. Heywang-Köbrunner SH, Sinnatamby R, Lebeau A, Lebrecht A,
Britton PD, Schreer I, et al. Interdisciplinary consensus on the
uses and technique of MR-guided vacuum-assisted breast biopsy
(VAB): results of a European consensus meeting. Eur J Radiol.
2009;72:289-94. Crossref
10. American College of Radiology. Breast Imaging Reporting and Data System, 5th edition. Reston, VA: American College of Radiology; 2013.
11. Niketa C, Pang KA, Lim JW. Challenges in MRI-guided breast biopsy and some suggested strategies: case-based review. Diagnostics (Basel). 2022;12:1985. Crossref
12. Chesebro AL, Chikarmane SA, Ritner JA, Birdwell RL, Giess CS. Troubleshooting to overcome technical challenges in image-guided breast biopsy. Radiographics. 2017;37:705-18. Crossref
13. Gao P, Kong X, Song Y, Song Y, Fang Y, Ouyang H, et al. Recent progress for the techniques of MRI-guided breast interventions
and their applications on surgical strategy. J Cancer. 2020;11:4671-82. Crossref
14. Brennan SB, Sung JS, Dershaw DD, Liberman L, Morris EA.
Cancellation of MR imaging–guided breast biopsy due to
lesion nonvisualization: frequency and follow-up. Radiology.
2011;261:92-9. Crossref
15. Meucci R, Pistolese Chiara A, Perretta T, Vanni G, Portarena I,
Manenti G, et al. MR imaging–guided vacuum assisted breast
biopsy: radiological-pathological correlation and underestimation
rate in pre-surgical assessment. Eur J Radiol Open. 2020;7:100244. Crossref
16. Myers KS, Kamel IR, Macura KJ. MRI-guided breast biopsy:
outcomes and effect on patient management. Clin Breast Cancer.
2015;15:143-52. Crossref