Uses of Contrast-Enhanced Mammography in a Regional Clinical Institute: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2025;28:Epub 11 September 2025
Uses of Contrast-Enhanced Mammography in a Regional Clinical
Institute: A Pictorial Essay
CN Hui, BTY Ko, CKM Mo, AYT Lai, WWC Wong
Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr CN Hui, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email: hcn010@ha.org.hk
Submitted: 8 January 2024; Accepted: 27 August 2024. This version may differ from the final version when published in an issue.
Contributors: CNH and AYTL designed the study, acquired and analysed the data. CNH drafted the manuscript. All authors critically revised
the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by Hospital Authority Central Institutional Review Board, Hong Kong (Ref No.: CIRB-2024-005-4).
Informed patient consent was waived by the Board as this article used anonymised patient data and posed no more than minimal risk.
INTRODUCTION
Contrast-enhanced mammography (CEM) is a modality
used as an adjunct to non-contrast mammographic imaging
for multiple clinical indications, including evaluation of
indeterminate abnormalities on mammography, workup
of symptomatic patients, staging of breast cancer, and
monitoring response to neoadjuvant chemotherapy.
Our centre provides diagnostic imaging for patients with
breast symptoms through combined full-field digital
mammography with tomosynthesis and ultrasound. We
introduced CEM in 2019. This pictorial essay highlights
the uses of CEM in daily clinical practice through
selected cases.
IMAGING PROTOCOL
We use a Selenia Dimensions 3D Digital Mammography
system (Hologic, Glasgow [DE], US) to acquire CEM
images. Two minutes prior to image acquisition, iohexol
(Omnipaque 300; GE Healthcare, Milwaukee [WI], US)
is administered intravenously at 1.5 mL/kg and a rate of
3 mL/s, followed by a saline flush. The breasts are not
compressed during injection to facilitate blood flow.
A pair of images (one low-energy image and one high-energy
image) is acquired in the standard craniocaudal
and mediolateral oblique views for each breast. Additional
views, including magnification or spot compression, can
be acquired if needed using conventional mammographic
technique. The ideal imaging window is within 10
minutes after contrast administration before contrast
washout commences.[1] [2]
The low-energy images used in subtraction have been
shown to have equivalent diagnostic value compared to
full-field digital mammography, as their K-edge is lower
than that of iodine, eliminating the need for an additional
set of conventional images.[3] These images are reported
using the latest BI-RADS (Breast Imaging Reporting and
Data System) mammography lexicon.[4] The high-energy
images are used to subtract the low-energy images,
emphasising the areas of iodine uptake. The subtracted
images are interpreted using the BI-RADS 2022 CEM
lexicon.[5]
There is currently no universal consensus on the
imaging sequence.[1] Our centre prefers to start with the symptomatic breast as it presumably has the highest
concentration of contrast agent and, therefore, better
lesion conspicuity at the beginning of image acquisition.[2]
No consensus has been reached regarding the optimal
timing of CEM within the menstrual cycle.[6] [7]
DIAGNOSTIC PERFORMANCE OF CONTRAST-ENHANCED MAMMOGRAPHY
Full-field digital mammography and/or tomosynthesis
and ultrasound remain the mainstays of breast assessment.
However, the sensitivity of mammography decreases in
dense breast parenchyma. Magnetic resonance imaging
(MRI) is an advanced imaging modality known for its
high sensitivity and negative predictive value (NPV),
though its use is compromised by high cost and limited
availability.
CEM, a relatively recent and more affordable adjunct
for assessing both lesion morphology and vascularity,
has gained popularity. Various studies have evaluated
its diagnostic performance. CEM has been shown to
have superior clinical performance compared to full-field
digital mammography and/or ultrasound.[8] [9] [10] Cheung et al[8] showed that CEM increased sensitivity from
71.5% to 92.7% and specificity from 51.8% to 67.9%
in dense breasts, compared to mammography alone.
In a meta-analysis by Cozzi et al,[11] CEM had a pooled
sensitivity of 95% and specificity of 81% in breast cancer
detection. CEM shares MRI’s sensitivity for diagnosing
breast cancer.[12] [13] It may be a reliable alternative when
MRI is contraindicated or not tolerated, or when
simultaneous assessment of suspicious calcifications
and contrast enhancement is needed. However, lesion
location may affect the feasibility of CEM as an
alternative. Lesions in obscured areas on conventional
mammography and nodal status are not well assessed by
CEM since they share same field of view and might be
overlooked.[14]
EVALUATION OF ARCHITECTURAL DISTORTION
The BI-RADS lexicon defines architectural distortion
(AD) as “a distortion of breast tissue with no definite
visible mass but with spiculations that radiate from a
point with focal retraction or distortion at the edge of
the parenchyma”.[4] Differential diagnoses include both
malignant and benign entities, such as radial scars,
complex sclerosing lesions, and postoperative changes.
Image-guided biopsy or surgical excision is typically
recommended for suspicious AD.
With the increasing use of digital breast tomosynthesis,
the detection rate of AD has risen.[15] One study reported
that nearly 60% of AD foci are benign.[16] Identifying
features that support or discourage biopsy may be
helpful. Contrast-enhanced modalities such as MRI
enable further evaluation of AD, especially if the finding
is equivocal, by assessing areas of contrast enhancement,
presumably related to angiogenesis and vascular leakage
in malignancies.[17] MRI has shown a high NPV (98%) when there is no enhancement in AD,[18] but it is not easily
accessible due to high costs and long queuing times in a
busy clinical institute setting.
CEM is a promising alternative for AD assessment,
with lower cost and shorter acquisition time. Patel et al[19]
reported a high NPV (92%) for malignancy when
primary AD showed no enhancement on CEM. While
this supports using CEM as an additional tool for
assessing tomosynthesis-detected AD, our centre still
recommends image-guided biopsy as the likelihood of
malignancy remains in BI-RADS category 4A lesions,
and some low-grade malignant lesions may not enhance.
Conversely, the high NPV in non-enhancing primary AD
could be applied in cases where a histological diagnosis
had already been obtained to help confirm imaging-histopathologic
concordance in cases with benign
biopsy results.[20] The increasing number of AD biopsies
yielding non-malignant pathology, due to the better
detection rate of AD on digital breast tomosynthesis,
adds complexity. The absence of contrast enhancement
on CEM can help confirm concordance in these cases
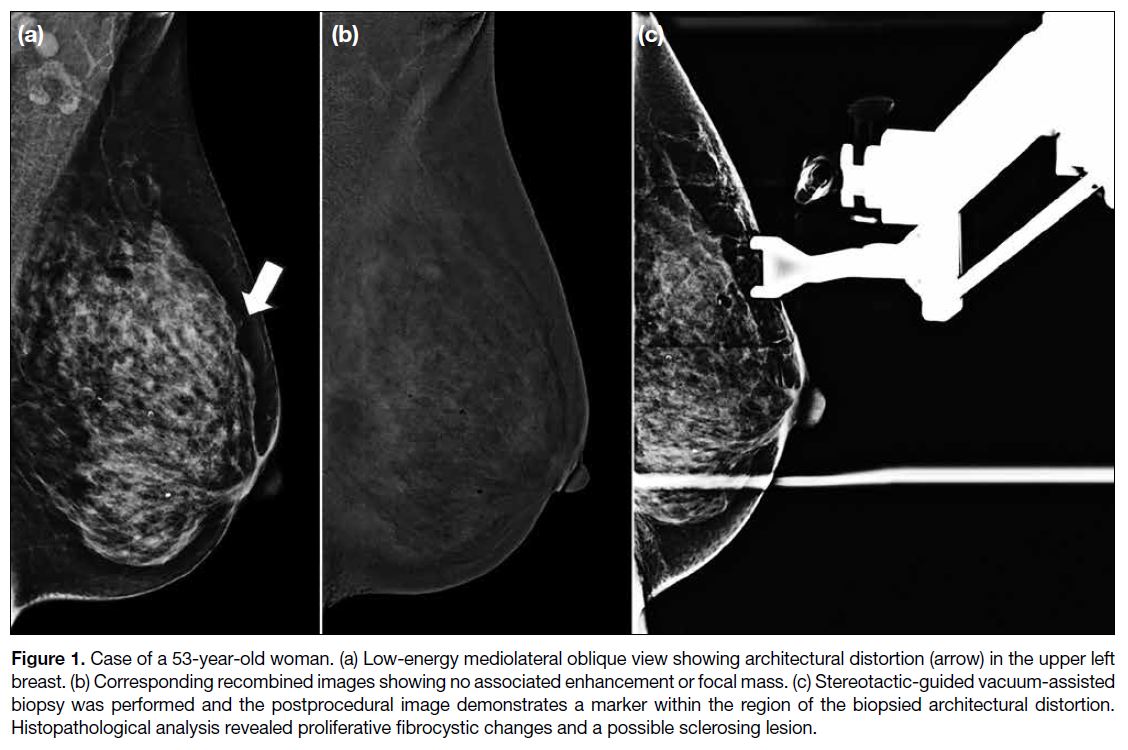
(Figure 1).
Figure 1. Case of a 53-year-old woman. (a) Low-energy mediolateral oblique view showing architectural distortion (arrow) in the upper left
breast. (b) Corresponding recombined images showing no associated enhancement or focal mass. (c) Stereotactic-guided vacuum-assisted
biopsy was performed and the postprocedural image demonstrates a marker within the region of the biopsied architectural distortion.
Histopathological analysis revealed proliferative fibrocystic changes and a possible sclerosing lesion.
ASSESSMENT OF CALCIFICATIONS
The approach to calcifications varies with the degree of
malignancy risk based on BI-RADS descriptors.[4] Biopsy
is offered for suspicious cases, while short-interval
follow-up imaging is recommended for those deemed
probably benign.
Sometimes suspicious calcifications can be challenging
to manage, especially when unaccompanied by
soft-tissue abnormalities and with no corresponding
sonographic lesion to allow further actions such as
localisation or biopsy in a readily feasible way. As CEM
consists of both low-energy and subtracted images, this
allows for the simultaneous delineation of calcifications
and associated contrast enhancement. The presence of
associated contrast enhancement correlates well with
the likelihood of malignancy.[21] [22] Therefore, it may be useful for selecting lesions for CEM-guided localisation,
particularly in cases of nonpalpable lesions that are
invisible on sonography (Figure 2). Again, however,
the absence of contrast enhancement does not exclude
malignancy.[21] [22] The morphology and distribution of
calcifications remain the key determinants. Further
exploration of this application is warranted.
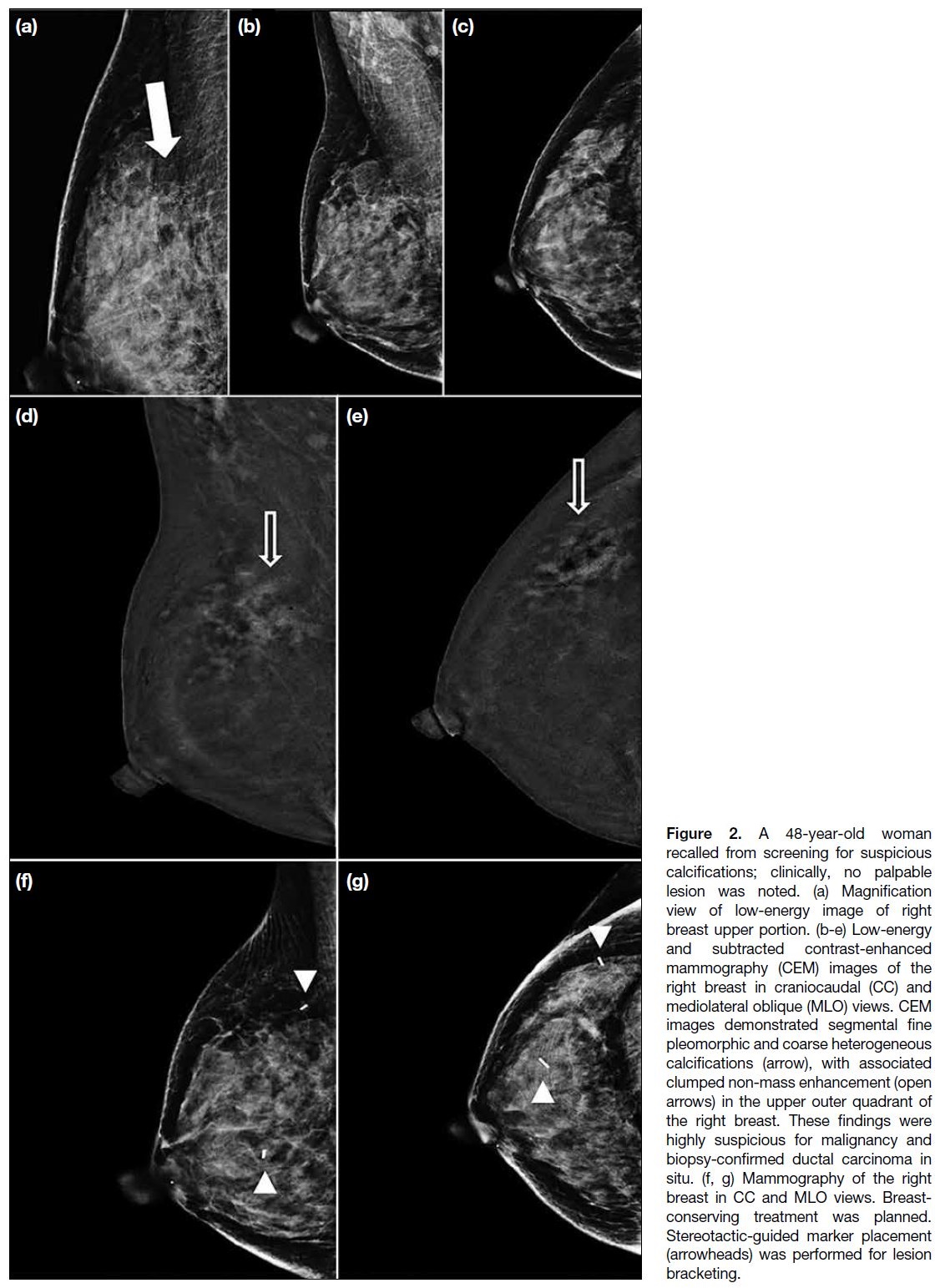
Figure 2. A 48-year-old woman
recalled from screening for suspicious
calcifications; clinically, no palpable
lesion was noted. (a) Magnification
view of low-energy image of right
breast upper portion. (b-e) Low-energy
and subtracted contrast-enhanced
mammography (CEM) images of the
right breast in craniocaudal (CC) and
mediolateral oblique (MLO) views. CEM
images demonstrated segmental fine
pleomorphic and coarse heterogeneous
calcifications (arrow), with associated
clumped non-mass enhancement (open
arrows) in the upper outer quadrant of
the right breast. These findings were
highly suspicious for malignancy and
biopsy-confirmed ductal carcinoma in
situ. (f, g) Mammography of the right
breast in CC and MLO views. Breast-conserving
treatment was planned.
Stereotactic-guided marker placement
(arrowheads) was performed for lesion
bracketing.
PREOPERATIVE STAGING OF BREAST CANCER
It is often difficult to determine the optimal surgical
approach, namely, breast-conserving treatment or
mastectomy, in patients with suspected additional
tumour foci in the ipsilateral or contralateral breast. In
addition, the Asian population often has dense breast
tissue,[23] which lowers the sensitivity of mammography
and complicates the surgical decision making.
The application of CEM in assessing tumour extent
has been compared to conventional mammography,
ultrasound, and MRI. Both CEM and MRI have higher
sensitivity for cancer detection compared to conventional
mammography and ultrasound alone (Figures 3, 4, 5, and 6).[24] [25]
Compared to MRI, CEM exhibits similar sensitivity in detecting the index cancer and secondary cancer.[25] [26]
Preliminary results from the studies[24] [25] [26] demonstrate that
CEM may be a feasible and cost-effective modality for
preoperative staging.
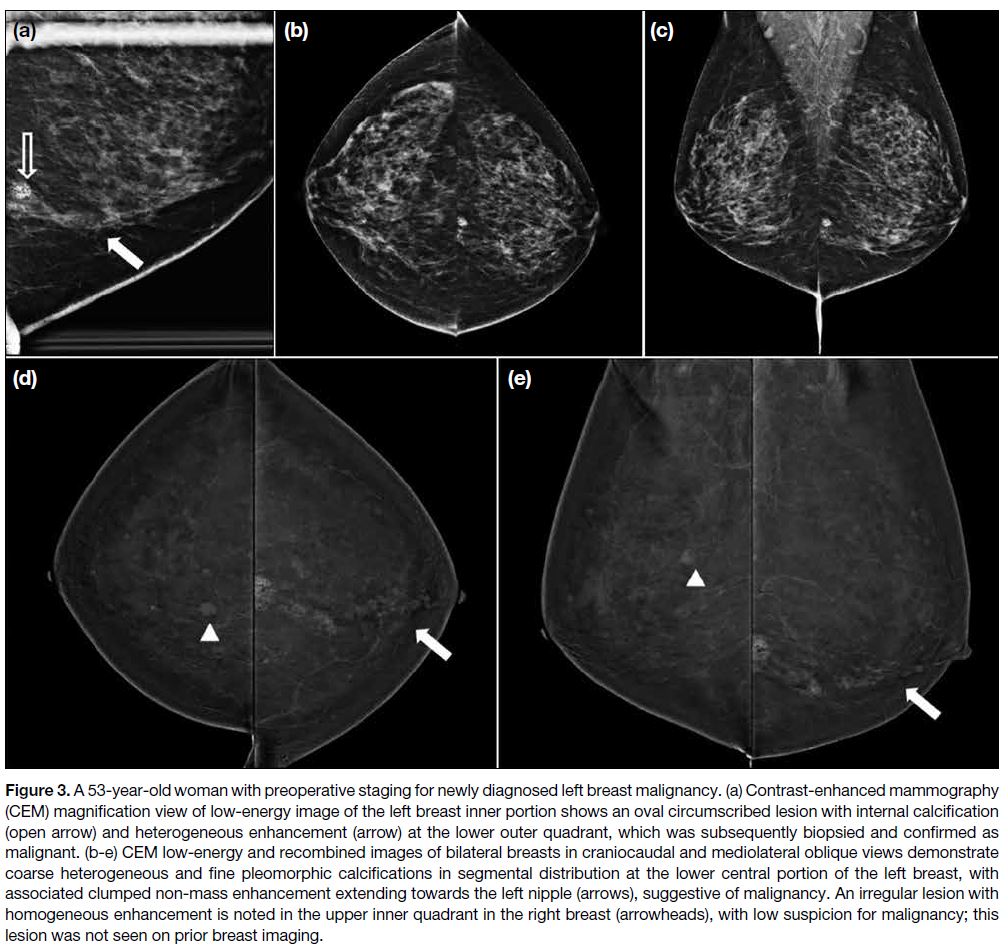
Figure 3. A 53-year-old woman with preoperative staging for newly diagnosed left breast malignancy. (a) Contrast-enhanced mammography
(CEM) magnification view of low-energy image of the left breast inner portion shows an oval circumscribed lesion with internal calcification
(open arrow) and heterogeneous enhancement (arrow) at the lower outer quadrant, which was subsequently biopsied and confirmed as
malignant. (b-e) CEM low-energy and recombined images of bilateral breasts in craniocaudal and mediolateral oblique views demonstrate
coarse heterogeneous and fine pleomorphic calcifications in segmental distribution at the lower central portion of the left breast, with
associated clumped non-mass enhancement extending towards the left nipple (arrows), suggestive of malignancy. An irregular lesion with
homogeneous enhancement is noted in the upper inner quadrant in the right breast (arrowheads), with low suspicion for malignancy; this
lesion was not seen on prior breast imaging.
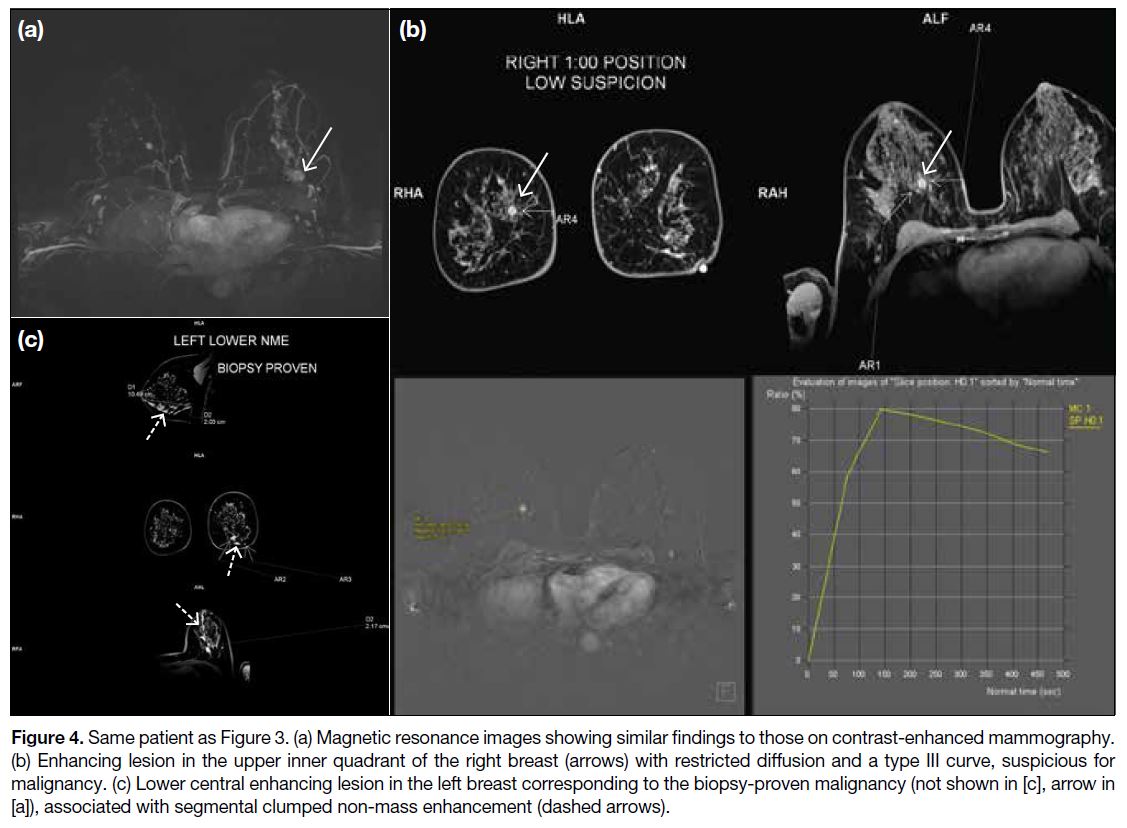
Figure 4. Same patient as Figure 3. (a) Magnetic resonance images showing similar findings to those on contrast-enhanced mammography.
(b) Enhancing lesion in the upper inner quadrant of the right breast (arrows) with restricted diffusion and a type III curve, suspicious for
malignancy. (c) Lower central enhancing lesion in the left breast corresponding to the biopsy-proven malignancy (not shown in [c], arrow in
[a]), associated with segmental clumped non-mass enhancement (dashed arrows).
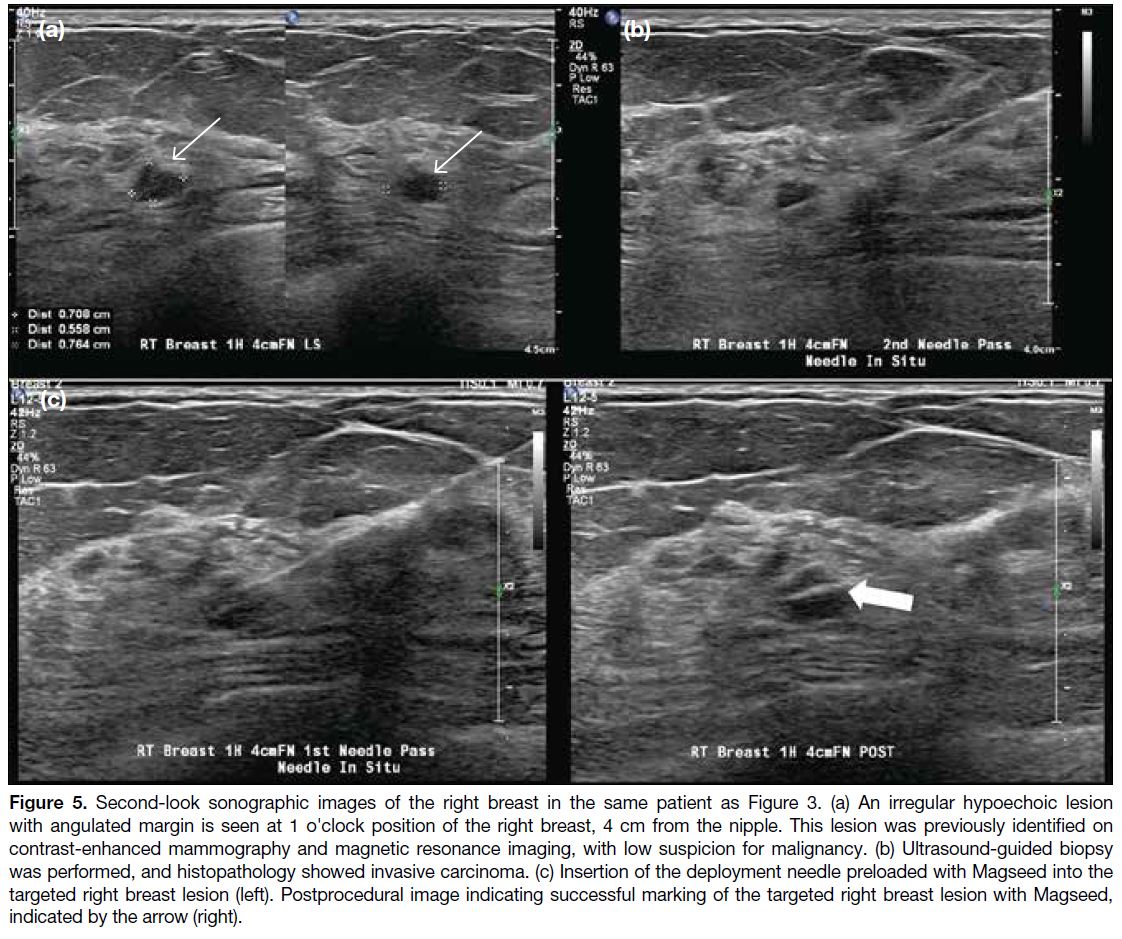
Figure 5. Second-look sonographic images of the right breast in the same patient as Figure 3. (a) An irregular hypoechoic lesion
with angulated margin is seen at 1 o'clock position of the right breast, 4 cm from the nipple. This lesion was previously identified on
contrast-enhanced mammography and magnetic resonance imaging, with low suspicion for malignancy. (b) Ultrasound-guided biopsy
was performed, and histopathology showed invasive carcinoma. (c) Insertion of the deployment needle preloaded with Magseed into the
targeted right breast lesion (left). Postprocedural image indicating successful marking of the targeted right breast lesion with Magseed,
indicated by the arrow (right).
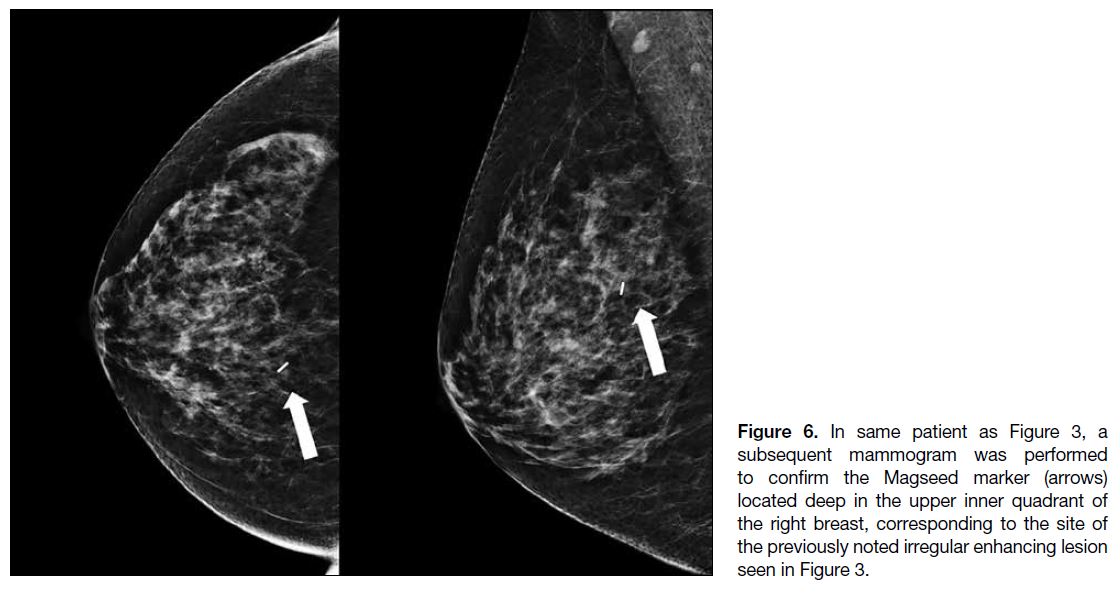
Figure 6. In same patient as Figure 3, a
subsequent mammogram was performed
to confirm the Magseed marker (arrows)
located deep in the upper inner quadrant of
the right breast, corresponding to the site of
the previously noted irregular enhancing lesion
seen in Figure 3.
MONITORING RESPONSE TO NEOADJUVANT CHEMOTHERAPY
Neoadjuvant chemotherapy (NAC) has been an
important strategy for patients with locally advanced
breast cancer. NAC helps improve surgical and cosmetic
outcomes by shrinking tumour size, downgrading the
nodal status, and increasing the likelihood of successful
breast conservation.
Accurate imaging assessment of treatment response is
therefore essential, and MRI is a reliable tool, superior
to the combination of clinical examination, conventional
mammography, and ultrasound.[27] Nonetheless, MRI is
not always readily available due to limited access and
patient-related factors such as long wait times, allergy
to contrast agents, kidney problems, claustrophobia,
or the presence of metallic devices (Figures 7 and 8).
CEM has a lower cost in terms of examination time
and resources; it has been an increasingly popular tool to assess treatment response. The results are promising,
showing comparable performance between MRI and
CEM in evaluating the pathological response of breast
cancer to NAC (Figures 9 and 10).[28]
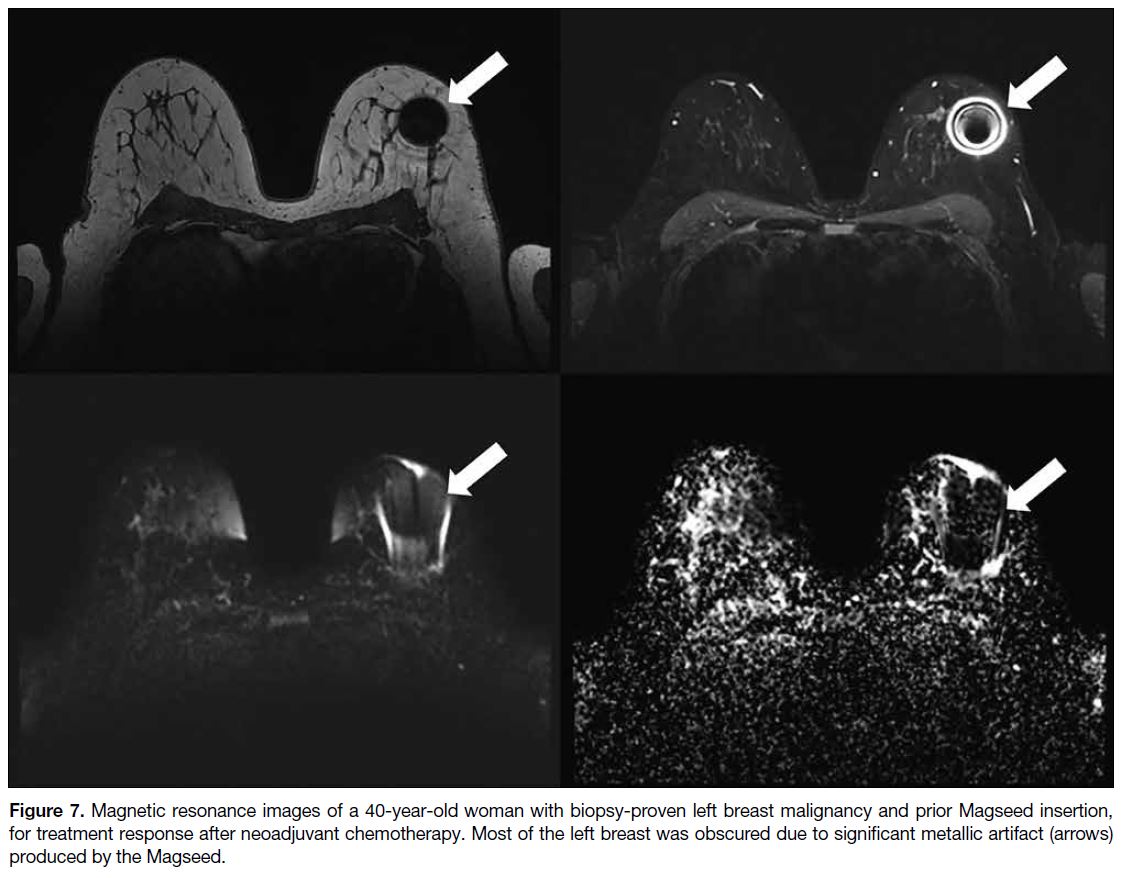
Figure 7. Magnetic resonance images of a 40-year-old woman with biopsy-proven left breast malignancy and prior Magseed insertion,
for treatment response after neoadjuvant chemotherapy. Most of the left breast was obscured due to significant metallic artifact (arrows)
produced by the Magseed.
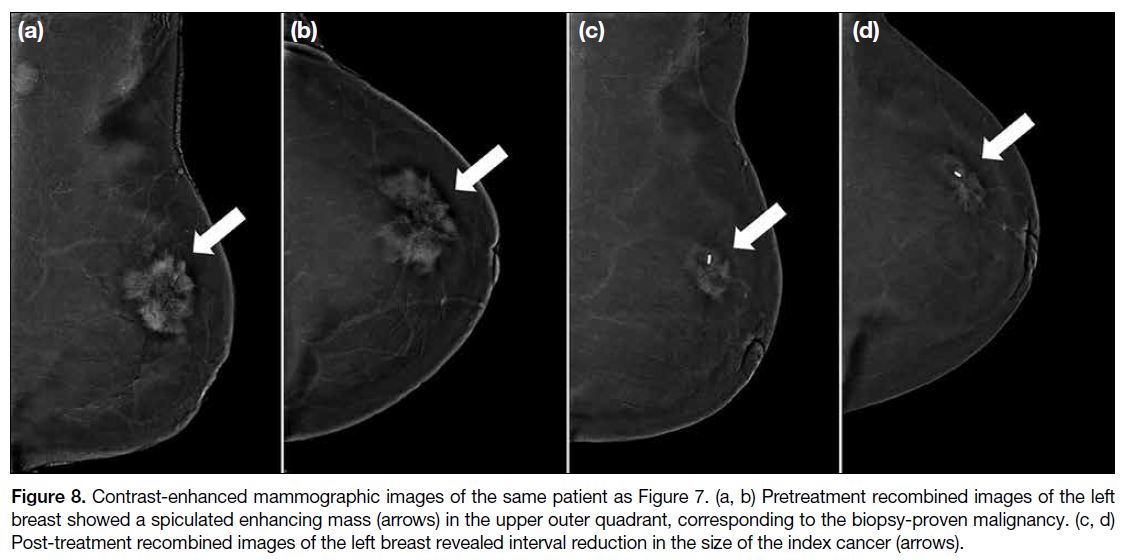
Figure 8. Contrast-enhanced mammographic images of the same patient as Figure 7. (a, b) Pretreatment recombined images of the left
breast showed a spiculated enhancing mass (arrows) in the upper outer quadrant, corresponding to the biopsy-proven malignancy. (c, d)
Post-treatment recombined images of the left breast revealed interval reduction in the size of the index cancer (arrows).
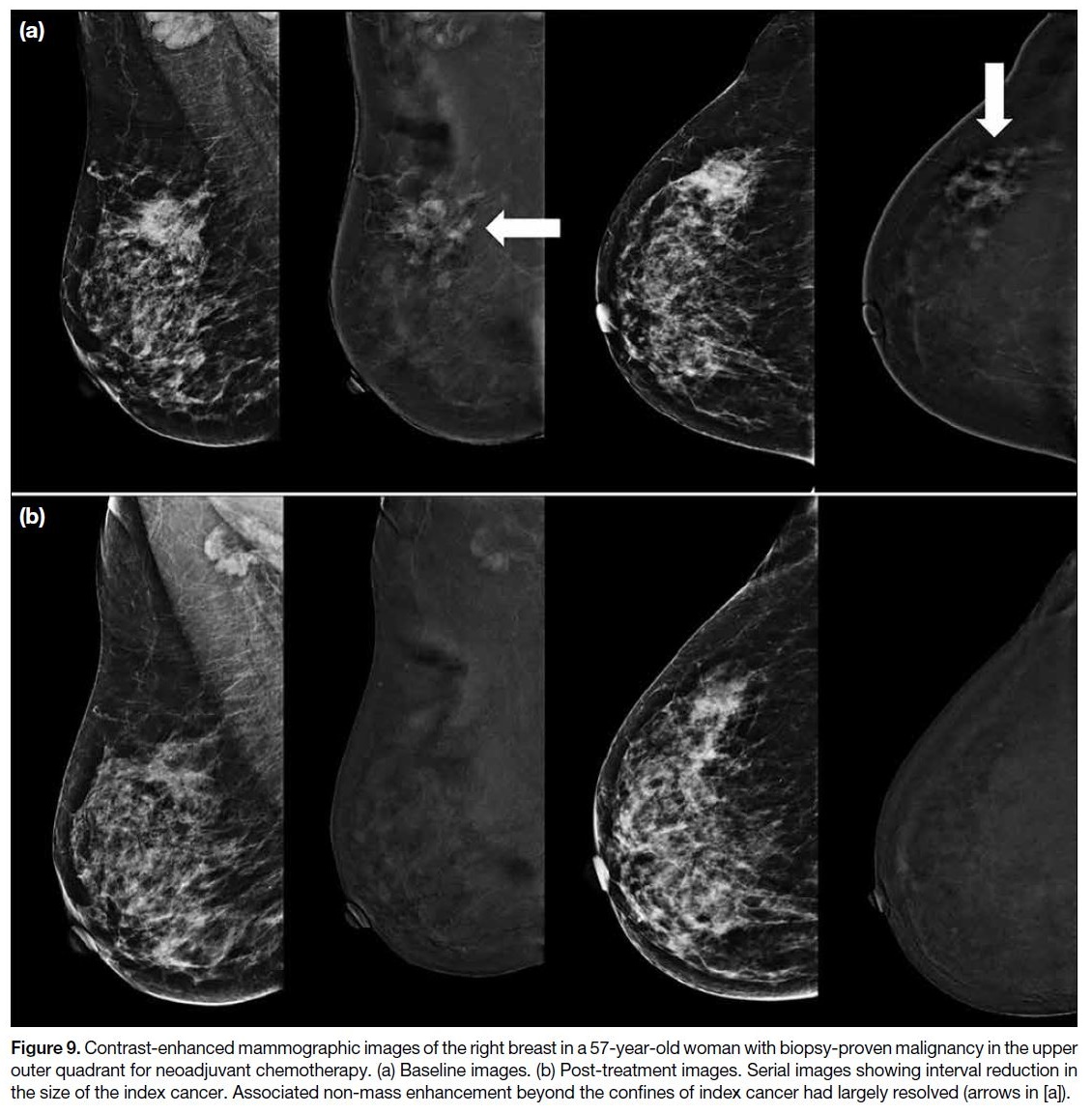
Figure 9. Contrast-enhanced mammographic images of the right breast in a 57-year-old woman with biopsy-proven malignancy in the upper
outer quadrant for neoadjuvant chemotherapy. (a) Baseline images. (b) Post-treatment images. Serial images showing interval reduction in
the size of the index cancer. Associated non-mass enhancement beyond the confines of index cancer had largely resolved (arrows in [a]).
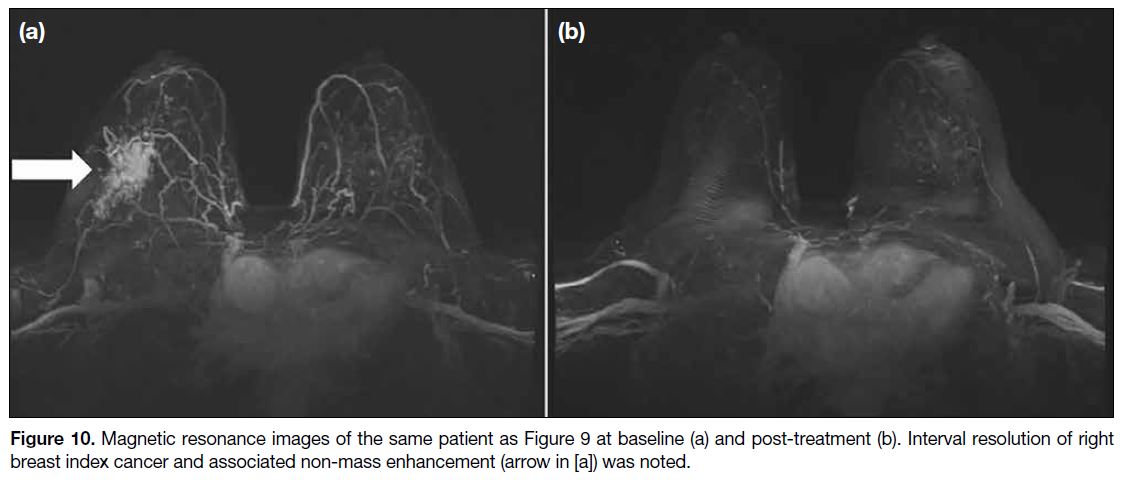
Figure 10. Magnetic resonance images of the same patient as Figure 9 at baseline (a) and post-treatment (b). Interval resolution of right
breast index cancer and associated non-mass enhancement (arrow in [a]) was noted.
OTHER CONSIDERATIONS: BREAST
CANCER SCREENING
MRI is recommended as a supplemental screening tool
in breast cancer screening for high-risk populations,
defined as women with a lifetime risk of more than
20% according to the American Cancer Society and
the American College of Radiology.[29] For women at
intermediate risk, defined as those with a lifetime risk between 15% and 20%, breast MRI is suggested for
those with dense breasts and a history of breast cancer
diagnosed before the age of 50 years, according to the
American College of Radiology.[29] The introduction of
CEM has aroused radiologists’ interest in its role in
screening and surveillance, particularly given the large
number of intermediate-risk women who could benefit.
In a pilot study by Jochelson et al,[30] 307 patients at
increased risk for breast cancer underwent both screening
CEM and MRI. Both modalities detected additional
invasive cancers that were occult on conventional
mammography, with comparable specificity and
positive predictive value.[30] Two other studies showed that CEM outperformed two-dimensional full-field
digital mammography when screening women with
higher-than-average risk for breast cancer, with greater
sensitivity (e.g., 87.5% vs. 50%,[31] 90.5% vs. 52.4%[32]).
Preliminary study results are encouraging but the role of
CEM warrants further research.
LIMITATIONS AND PITFALLS OF CONTRAST-ENHANCED MAMMOGRAPHY
Adverse reaction to iodinated contrast agent is a concern,
including the risks of extravasation, allergy, and contrast-induced
acute kidney injury. Volume expansion by 0.9%
normal saline prior to the contrast administration is a
feasible preventive measure for those at risk.[33] The image
quality of CEM can be degraded by patient motion.
CEM is more prone to motion artifacts (Figure 11) due
to its longer exposure and compression time, resulting in
blurred images. There are many technical artifacts that are
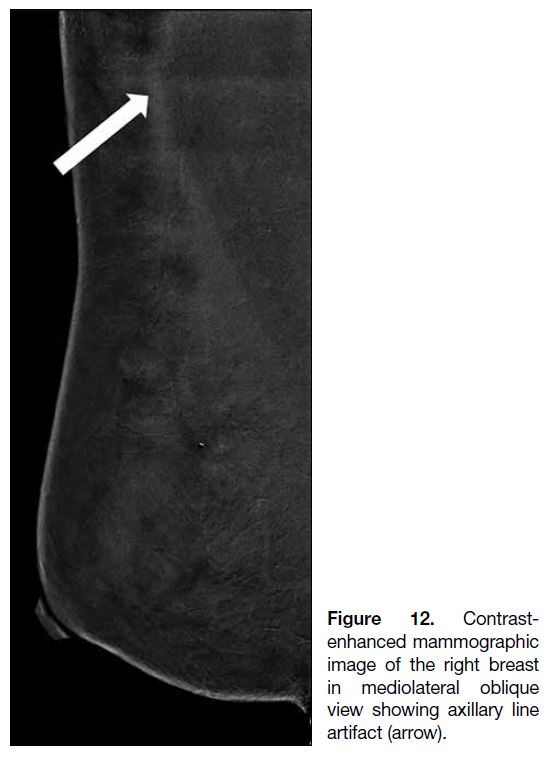
specific to CEM. For instance, the use of an undersized
compression paddle may cause horizontal lines across the axilla (Figure 12). Suboptimal breast compression may
lead to air trapping within skin folds or scars, resulting
in poor contact between the skin and the detector or
compression paddle (Figure 13). Macrocalcifications,
cysts or, post-biopsy haematomas may not enhance and
appear as low-density areas compared to background
enhancement on subtracted images, which is known as
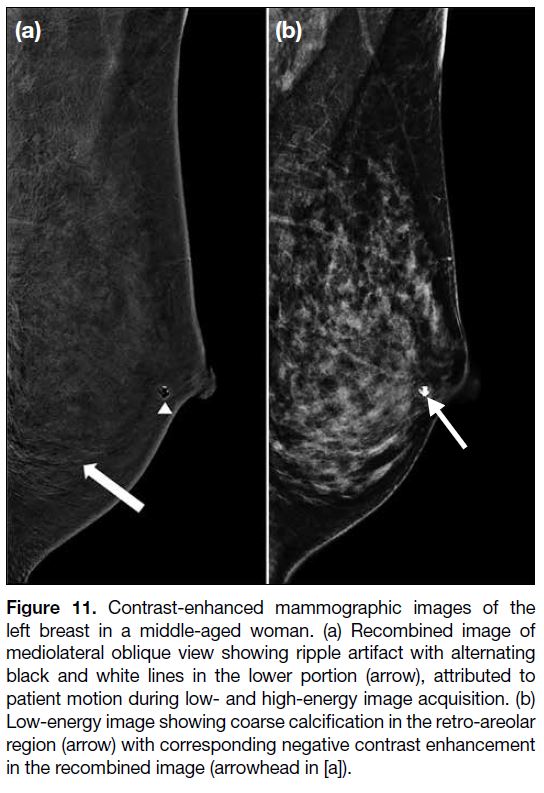
negative contrast enhancement[14] [34] (Figure 11).
Figure 11. Contrast-enhanced mammographic images of the
left breast in a middle-aged woman. (a) Recombined image of
mediolateral oblique view showing ripple artifact with alternating
black and white lines in the lower portion (arrow), attributed to
patient motion during low- and high-energy image acquisition. (b)
Low-energy image showing coarse calcification in the retro-areolar
region (arrow) with corresponding negative contrast enhancement
in the recombined image (arrowhead in [a]).
Figure 12. Contrast-enhanced
mammographic image of the right breast in mediolateral oblique view showing axillary line
artifact (arrow).
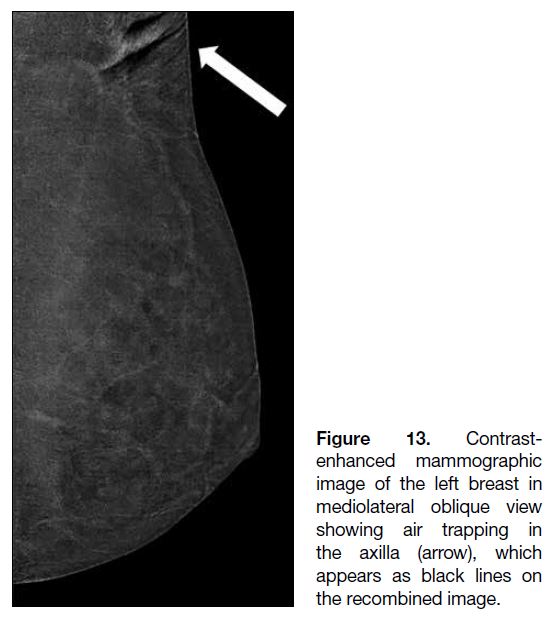
Figure 13. Contrast-enhanced
mammographic image of the left breast in
mediolateral oblique view showing air trapping in
the axilla (arrow), which appears as black lines on the recombined image.
Subtracted CEM allows delineation of contrast
enhancement based on the degree of angiogenesis in the
lesions. However, contrast enhancement may also be
seen in benign lesions such as fibroadenomas, intraductal
papillomas, and fat necrosis,[14] making it difficult to
determine the nature of the lesion and potentially
resulting in false-positive findings. Varying degrees of
angiogenesis and contrast enhancement are also seen
among different subtypes of malignancy. Lesions with
less pronounced enhancement, such as ductal carcinoma
in situ or lobular carcinoma, may be overlooked,[14]
leading to false-negative findings.
Additionally, the intensity of background parenchymal
enhancement can affect interpretation, as it may obscure
underlying lesions[14] (Figure 14). Since the field of view in
CEM is identical to that of conventional mammography,
lesions located in blind spots, such as those near the chest
wall, might not be visualised[14] and should be further
evaluated with MRI.
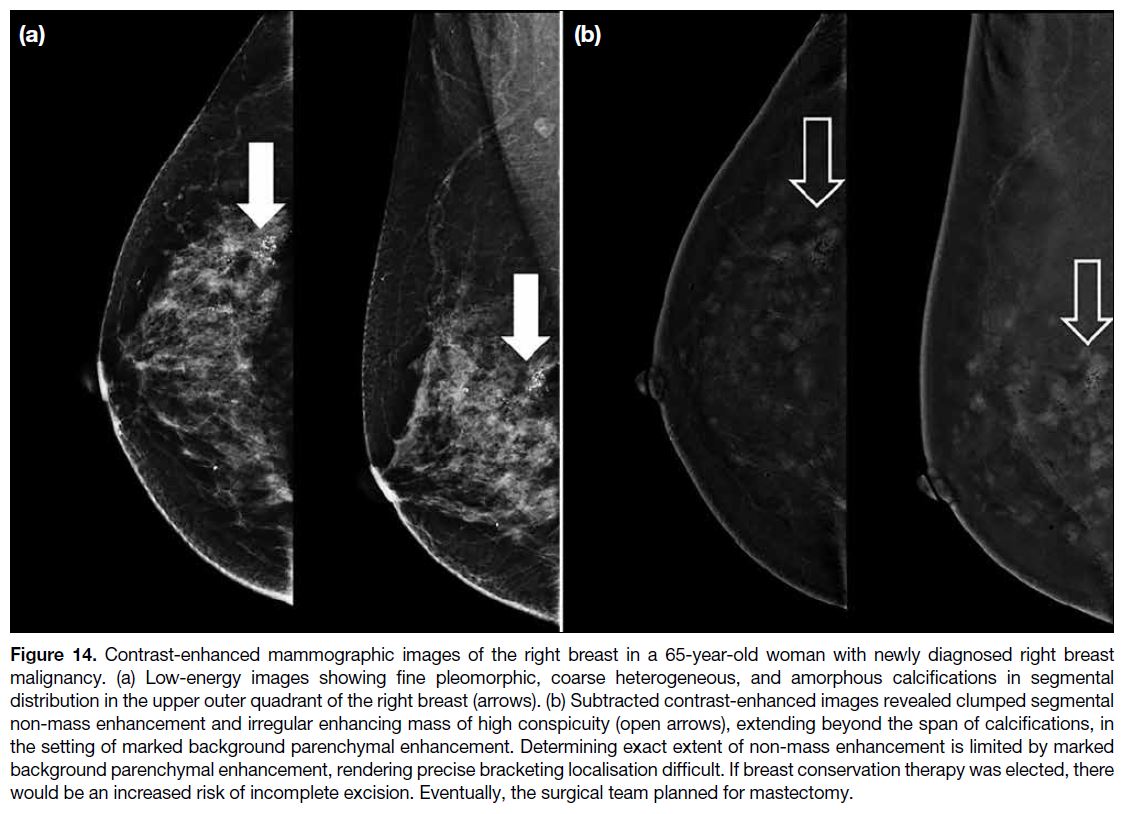
Figure 14. Contrast-enhanced mammographic images of the right breast in a 65-year-old woman with newly diagnosed right breast
malignancy. (a) Low-energy images showing fine pleomorphic, coarse heterogeneous, and amorphous calcifications in segmental
distribution in the upper outer quadrant of the right breast (arrows). (b) Subtracted contrast-enhanced images revealed clumped segmental
non-mass enhancement and irregular enhancing mass of high conspicuity (open arrows), extending beyond the span of calcifications, in
the setting of marked background parenchymal enhancement. Determining exact extent of non-mass enhancement is limited by marked
background parenchymal enhancement, rendering precise bracketing localisation difficult. If breast conservation therapy was elected, there
would be an increased risk of incomplete excision. Eventually, the surgical team planned for mastectomy.
Currently, there are not good biopsy tools that work
directly with CEM. Lesions identified on CEM should be correlated with other imaging modalities (e.g., standard
digital mammography, ultrasound, or breast MRI) if
biopsy is planned. Ultrasound is often preferred due to its
accessibility, lower cost, and suitability for ultrasoundguided
biopsy.
CONCLUSION
Combined full-field digital mammography with
tomosynthesis and ultrasound remains the mainstay of
breast assessment in our centre. Incorporation of CEM into daily clinical practice provides further additional
information in certain circumstances and is commonly
used for evaluating disease extent and monitoring
treatment response. CEM is increasingly regarded as a more affordable and accessible modality in settings
with limited resources, or as an alternative when MRI is
contraindicated or not tolerated. Further exploration of
its role in breast imaging is anticipated.
REFERENCES
1. Perry H, Phillips J, Dialani V, Slanetz PJ, Fein-Zachary VJ,
Karimova EJ, et al. Contrast-enhanced mammography: a systematic
guide to interpretation and reporting. AJR Am J Roentgenol.
2019;212:222-31. Crossref
2. Smith A. The principles of contrast mammography. Available from:
https://www.hologic.com/sites/default/files/The%20Principles%20of%20Contrast%20Mammography.pdf. Accessed 16 Nov 2023.
3. Francescone MA, Jochelson MS, Dershaw DD, Sung JS, Hughes MC, Zheng J, et al. Low energy mammogram obtained in contrast-enhanced
digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol. 2014;83:1350-5. Crossref
4. D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS® Atlas: Breast Imaging Reporting and Data System. Reston
(VA): American College of Radiology; 2013.
5. Lee CH, Phillips J, Sung JS, Lewin JM, Newell MS. Contrast
Enhanced Mammography (CEM) (A supplement to ACR
BI-RADS® Mammography 2013). 2022. Available from:
https://edge.sitecorecloud.io/americancoldf5f-acrorgf92a-productioncb02-3650/media/ACR/Files/RADS/BI-RADS/Contrast-Enhanced-Mammography-Supplement.pdf. Accessed 16 Nov 2023.
6. Karimi Z, Phillips J, Slanetz P, Lotfi P, Dialani V, Karimova J, et al. Factors associated with background parenchymal enhancement on contrast-enhanced mammography. AJR Am J Roentgenol.
2021;216:340-8. Crossref
7. Sogani J, Morris EA, Kaplan JB, D’Alessio D, Goldman D, Moskowitz CS, et al. Comparison of background parenchymal
enhancement at contrast-enhanced spectral mammography and
breast MR imaging. Radiology. 2017;282:63-73. Crossref
8. Cheung YC, Lin YC, Wan YL, Yeow KM, Huang PC, Lo YF, et al. Diagnostic performance of dual-energy contrast-enhanced
subtracted mammography in dense breasts compared to
mammography alone: interobserver blind-reading analysis. Eur
Radiol. 2014;24:2394-403. Crossref
9. Luczyńska E, Heinze S, Adamczyk A, Rys J, Mitus JW, Hendrick E. Comparison of the mammography, contrast-enhanced spectral
mammography and ultrasonography in a group of 116 patients.
Anticancer Res. 2016;36:4359-66.
10. Mori M, Akashi-Tanaka S, Suzuki S, Daniels MI, Watanabe C, Hirose M, et al. Diagnostic accuracy of contrast-enhanced spectral
mammography in comparison to conventional full-field digital
mammography in a population of women with dense breasts. Breast
Cancer. 2017;24:104-10. Crossref
11. Cozzi A, Magni V, Zanardo M, Schiaffino S, Sardanelli F. Contrast-enhanced
mammography: a systematic review and meta-analysis
of diagnostic performance. Radiology. 2022;302:568-81. Crossref
12. Gelardi F, Ragaini EM, Sollini M, Bernardi D, Chiti A. Contrast-enhanced
mammography versus breast magnetic resonance
imaging: a systematic review and meta-analysis. Diagnostics
(Basel). 2022;12:1890. Crossref
13. Xiang W, Rao H, Zhou L. A meta-analysis of contrast-enhanced
spectral mammography versus MRI in the diagnosis of breast
cancer. Thorac Cancer. 2020;11:1423-32. Crossref
14. Lorente-Ramos RM, Azpeitia-Armán J, Oliva-Fonte C, Pérez-Bartolomé A, Azpeitia Hernández J. Contrast-enhanced
mammography artifacts and pitfalls: tips and tricks to avoid
misinterpretation Radiographics. 2023;43:e230021. Crossref
15. Partyka L, Lourenco AP, Mainiero MB. Detection of
mammographically occult architectural distortion on digital breast
tomosynthesis screening: initial clinical experience. AJR Am J
Roentgenol. 2014;203:216-22. Crossref
16. Ray KM, Turner E, Sickles EA, Joe BN. Suspicious findings
at digital breast tomosynthesis occult to conventional digital
mammography: imaging features and pathology findings. Breast
J. 2015;21:538-42. Crossref
17. Kuhl CK. MRI of breast tumors. Eur Radiol. 2000;10:46-58. Crossref
18. Niell BL, Bhatt K, Dang P, Humphrey K. Utility of breast MRI for further evaluation of equivocal findings on digital breast
tomosynthesis. AJR Am J Roentgenol. 2018;211:1171-8. Crossref
19. Patel BK, Naylor ME, Kosiorek HE, Lopez-Alvarez YM, Miller AM, Pizzitola VJ, et al. Clinical utility of contrast-enhanced spectral
mammography as an adjunct for tomosynthesis-detected
architectural distortion. Clin Imaging. 2017;46:44-52. Crossref
20. Goh Y, Quek ST, Pillay P, Chou CP. Evaluation of architectural distortion with contrast-enhanced mammography. Clin Radiol.
2024;79:163-9. Crossref
21. Cheung YC, Juan YH, Lin YC, Lo YF, Tsai HP, Ueng SH, et al. Dual-energy contrast-enhanced spectral mammography: enhancement analysis on BI-RADS 4 non-mass microcalcifications
in screened women. PLoS ONE. 2016;11:e0162740. Crossref
22. Houben IP, Vanwetswinkel S, Kalia V, Thywissen T, Nelemans PJ, Heuts EM, et al. Contrast-enhanced spectral mammography in the
evaluation of breast suspicious calcifications: diagnostic accuracy
and impact on surgical management. Acta Radiol. 2019;60:1110-7. Crossref
23. Habel LA, Capra AM, Oestreicher N, Greendale GA, Cauley JA,
Bromberger J, et al. Mammographic density in a multiethnic cohort.
Menopause. 2007;14:891-9. Crossref
24. Dromain C, Thibault F, Muller S, Rimareix F, Delaloge S, Tardivon A, et al. Dual-energy contrast-enhanced digital
mammography: initial clinical results. Eur Radiol. 2011;21:565-74. Crossref
25. Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital
mammography: feasibility and comparison with conventional
digital mammography and MR imaging in women with known
breast carcinoma. Radiology. 2013;266:743-51. Crossref
26. Lee-Felker SA, Tekchandani L, Thomas M, Gupta E, Andrews-Tang D, Roth A, et al. Newly diagnosed breast cancer: comparison
of contrast-enhanced spectral mammography and breast MR
imaging in the evaluation of extent of disease. Radiology.
2017;285:389-400. Crossref
27. Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M, Schipper R, et al. The role of magnetic resonance imaging in
assessing residual disease and pathologic complete response in
breast cancer patients receiving neoadjuvant chemotherapy: a
systematic review. Insights Imaging. 2013;4:163-75. Crossref
28. Tang S, Xiang C, Yang Q. The diagnostic performance of CESM
and CE-MRI in evaluating the pathological response to neoadjuvant
therapy in breast cancer: a systematic review and meta-analysis.
Br J Radiol. 2020;93:20200301. Crossref
29. Lee CS, Monticciolo DL, Moy L. Screening guidelines update for average-risk and high-risk women. AJR Am J Roentgenol.
2020;214:316-23. Crossref
30. Jochelson MS, Pinker K, Dershaw DD, Hughes M, Gibbons GF, Rahbar K, et al. Comparison of screening CEDM and MRI for
women at increased risk for breast cancer: a pilot study. Eur J
Radiol. 2017;97:37-43. Crossref
31. Sung JS, Lebron L, Keating D, D’Alessio D, Comstock CE,
Lee CH, et al. Performance of dual-energy contrast-enhanced
digital mammography for screening women at increased risk of
breast cancer. Radiology. 2019;293:81-8. Crossref
32. Sorin V, Yagil Y, Yosepovich A, Shalmon A, Gotlieb M, Neiman OH, et al. Contrast-enhanced spectral mammography in
women with intermediate breast cancer risk and dense breasts. AJR
Am J Roentgenol. 2018;211:W267-74. Crossref
33. ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media, American College of Radiology. 2023.
Available from: https://sar.org.ar/wp-content/uploads/2024/07/ACR2023Contrast_Media.pdf. Accessed 16 Nov 2023.
34. Nori J, Gill MK, Vignoli C, Bicchierai G, De Benedetto D, Di Naro F, et al. Artefacts in contrast enhanced digital
mammography: how can they affect diagnostic image quality and
confuse clinical diagnosis? Insights Imaging. 2020;11:16. Crossref