Outcomes of Peptide Receptor Radionuclide Therapy in Metastatic Neuroendocrine Tumours
ORIGINAL ARTICLE
Hong Kong J Radiol 2025;28:Epub 12 September 2025
Outcomes of Peptide Receptor Radionuclide Therapy in Metastatic Neuroendocrine Tumours
WH Wong1, HC Lam1, TK Au Yong2
1 Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Nuclear Medicine, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr WH Wong, Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: wwh986@ha.org.hk
Submitted: 28 June 2024; Accepted: 19 December 2024. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. WHW acquired and analysed the data and drafted the manuscript. All authors critically revised
the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, TKAY was not involved in the peer review process. Other authors have disclosed no conflicts of
interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Central Institutional Review Board of the Hospital Authority, Hong Kong (Ref No.: CIRB-2024-203-3) and was conducted according to the Declaration of Helsinki. The requirement for informed patient consent was waived by the
Board due to the retrospective nature of the research.
Abstract
Introduction
Peptide receptor radionuclide therapy (PRRT) using Lutetium-177 (177Lu) or Yttrium-90 (90Y) are
established treatments for metastatic neuroendocrine tumours (NETs). However, data on Chinese population remain
limited. This study aimed to examine the efficacy and safety of PRRT in Chinese patients with metastatic NETs.
Methods
We retrospectively analysed 21 Chinese patients with metastatic NETs treated with either 177Lu or a
combination of 177Lu and 90Y PRRT at Queen Elizabeth Hospital, Hong Kong, between 2018 and 2022. Tumour
response was evaluated using RECIST (Response Evaluation Criteria in Solid Tumors) 1.1. Kaplan–Meier analysis
was used to estimate progression-free survival (PFS) and overall survival (OS). Cox regression was used to identify
prognostic factors. Adverse events were graded using the Common Terminology Criteria for Adverse Events version 4.03.
Results
The most common primary tumour site was the pancreas (71.4%), followed by the rectum (23.8%) and
stomach (4.8%). 177Lu PRRT was used in 90.5% of cases, and a combination of 177Lu and 90Y in 9.5%. Treatment
results showed partial response in 47.6%, stable disease in 23.8%, and disease progression in 28.6%. Median PFS
was 22.3 months and median OS was 45.2 months. Multivariate analysis showed that bone metastasis significantly
worsened PFS (p = 0.02) and OS (p = 0.038), while a high liver metastatic burden (≥50% liver involvement) was
significantly associated with worse OS (p = 0.042).
Conclusion
PRRT is an effective and well-tolerated treatment for metastatic NETs in the Chinese population. Bone
metastases were associated with worse PFS and OS, while a high liver metastatic burden was associated with shorter
OS. These results can help clinicians in Hong Kong optimise patient selection and management strategies, though
larger prospective studies are needed to validate these findings.
Key Words: Gastrointestinal tract; Lutetium; Neuroendocrine Tumors; Progression-free survival; yttrium
中文摘要
轉移性神經內分泌腫瘤患者接受肽受體放射性核素治療的治療結果
黃偉軒、林河清、歐陽定勤
引言
肽受體放射性核素治療(PRRT)使用177鎦或90釔已被確立為治療轉移性神經內分泌腫瘤(NETs)的標準療法。然而,有關華人群體的數據仍然有限。本研究旨在評估PRRT在華籍轉移性NET患者中的療效與安全性。
方法
本研究回顧分析了2018年至2022年間,於香港伊利沙伯醫院接受177鎦或177鎦與90釔聯合PRRT治療的21位華籍轉移性NET患者。腫瘤反應根據實體腫瘤反應評估準則(RECIST)第1.1版進行評估。我們採用Kaplan–Meier方法估算無惡化存活期及總存活期,並使用Cox回歸分析找出預後因素。副作用根據美國國家癌症研究所通用不良事件術語標準(CTCAE)第4.03版進行分級。
結果
最常見的原發腫瘤部位為胰臟(71.4%),其次為直腸(23.8%)及胃部(4.8%)。90.5%患者接受177鎦治療,9.5%接受177鎦與90釔聯合治療。治療結果顯示47.6%達到部分緩解,23.8%為疾病穩定,28.6%為疾病惡化。中位無惡化存活期為22.3個月,中位總存活期為45.2個月。多變量分析顯示骨轉移與較差無惡化生存期(p = 0.02)及總存活期(p = 0.038)顯著相關;肝轉移負荷高(肝臟受累≥50%)亦與較短總生存期有顯著關聯(p = 0.042)。
結論
PRRT對華籍轉移性NET患者而言是一種有效且耐受性良好的治療方法。骨轉移與無惡化存活期及總存活期下降顯著相關,而高肝轉移負荷則與較短的總存活期有關。這些結果有助香港臨床醫生優化病人篩選及治療策略。不過,仍需進一步的大型前瞻性研究以驗證本研究的發現。
INTRODUCTION
Neuroendocrine tumours (NETs) are a heterogeneous
group of neoplasms originating from neuroendocrine
cells located in various anatomical sites, predominantly
the gastrointestinal (GI) tract, pancreas, and lungs.[1]
Approximately 20% of cases present with metastatic
disease at the time of diagnosis.[2] [3] For cases not amenable
to local treatment, systemic treatments commonly
include somatostatin analogues, targeted agents such
as sunitinib or everolimus, chemotherapy, and peptide
receptor radionuclide therapy (PRRT).[4] [5] [6]
PRRT exploits the high expression of somatostatin
receptors on NET cells,[7] enabling the targeted delivery
of radionuclides conjugated to somatostatin analogues.[8]
The two primary radionuclides used are Yttrium-90 (90Y)
and Lutetium-177 (177Lu). While 90Y emits high-energy
beta particles to induce cytotoxic effects, 177Lu emits
lower-energy beta particles with a shorter path length
of 1 to 2 mm, allowing more precise radiation delivery to
smaller metastases and reducing the overall toxicity.
The NETTER-1 phase 3 randomised trial demonstrated
that patients with well-differentiated, metastatic midgut NETs treated with 177Lu in combination with a
somatostatin analogue had a progression-free survival
(PFS) rate of 65.2% at 20 months, compared to 10.8%
in those receiving octreotide long-acting repeatable
alone.[9] The treatment was well tolerated, with significant
myelosuppression occurring in fewer than 10% of
patients and no observed renal toxicity during the
study period.[9] Subsequently, the US Food and Drug
Administration approved 177Lu-Dotatate in 2018 for the
treatment of somatostatin receptor–positive NETs, and it
has since become a standard of care in clinical practice.[10]
Despite the extensive data on PRRT from Europe and
the US,[11] [12] [13] [14] [15] there is a lack of local data for the Chinese
population. This retrospective study aimed to address this
gap by reporting treatment responses, survival outcomes,
and toxicity associated with PRRT in Chinese patients
treated at a hospital in Hong Kong.
METHODS
A retrospective cohort study was conducted on
patients with NETs treated with either 177Lu alone or
in combination with 90Y at the Department of Nuclear
Medicine, Queen Elizabeth Hospital, Hong Kong, between August 2018 and August 2022. The intended
PRRT regimen consisted of four to six cycles administered,
with 8 to 12 weeks between each cycle. An amino
acid infusion was given for renal protection, reducing
kidney radiation by limiting reabsorption and enhancing
clearance of the radiotracer. A post-therapy scan was
performed on day 4 following each treatment cycle.
Eligible patients were Chinese individuals aged 18 years
or above with histologically confirmed metastatic NETs.
All patients underwent either a baseline octreotide scan
or a 68Gallium-Dotatate positron emission tomography
scan to confirm somatostatin receptor expression, defined
as tumour uptake equal to or greater than that of normal
liver tissue. Patients receiving PRRT for paraganglioma
were excluded, as these tumours exhibit different
biological behaviour compared to epithelial NETs.
Electronic medical records were reviewed to extract
patient demographics, including sex, date of birth, date
of death (if applicable), date of NET diagnosis, primary
tumour location, World Health Organization grade, Ki-67
index, sites of metastases, hepatic metastatic burden, and
date of last follow-up. Data on previous treatments was
also collected, including surgical resection, locoregional
therapies such as transarterial chemoembolisation
and radiofrequency ablation, somatostatin analogues
(octreotide or lanreotide), targeted therapies (everolimus
or sunitinib), and chemotherapy (e.g., temozolomide or
capecitabine plus oxaliplatin). Laboratory parameters
were recorded before and after each course of PRRT,
including haemoglobin, neutrophil and lymphocyte
counts, creatinine levels, bilirubin, and alanine
transaminase levels. Symptoms after each course of
PRRT were extracted from the medical records.
Treatment response was assessed by comparing pre- and
post-PRRT imaging using RECIST (Response
Evaluation Criteria in Solid Tumors) 1.1. Survival
outcomes were analysed using Kaplan–Meier curves.
PFS was defined as the date from the first PRRT to
disease progression or death from any cause, and overall
survival (OS) was defined as the time from the first
PRRT to death. Toxicities were evaluated according to
the CTCAE (Common Terminology Criteria for Adverse
Events) version 4.03, with follow-up blood tests recorded at
each visit. Patients alive at the time of final analysis or
lost to follow-up were censored at the date they were last
known to be alive.
Two patients who demonstrated an initial response following their course of PRRT subsequently developed
disease progression and underwent retreatment with
PRRT. Retreatment PFS for these two patients was
presented separately and was not pooled with the cohort
PFS and OS analyses.
Normally distributed data are shown as means (standard
deviations), whereas non-normally distributed data
are shown as medians (ranges). Univariable Cox
proportional hazards regression was performed to assess
the association of baseline factors with PFS and OS.
Variables showing at least a trend towards significance
(p < 0.1) in the univariate analysis were subsequently
included in the multivariate analysis using the Cox
proportional hazards model. All tests were two-sided,
with a significance threshold of p < 0.05. Analyses were
conducted using SPSS (Windows version 24.0; IBM
Corp, Armonk [NY], US).
RESULTS
PRRT was performed on 23 Chinese patients between
August 2018 and August 2022 at Queen Elizabeth Hospital. Two patients were excluded from
this study because they had paragangliomas, leaving
a total of 21 patients included in the study. Patient
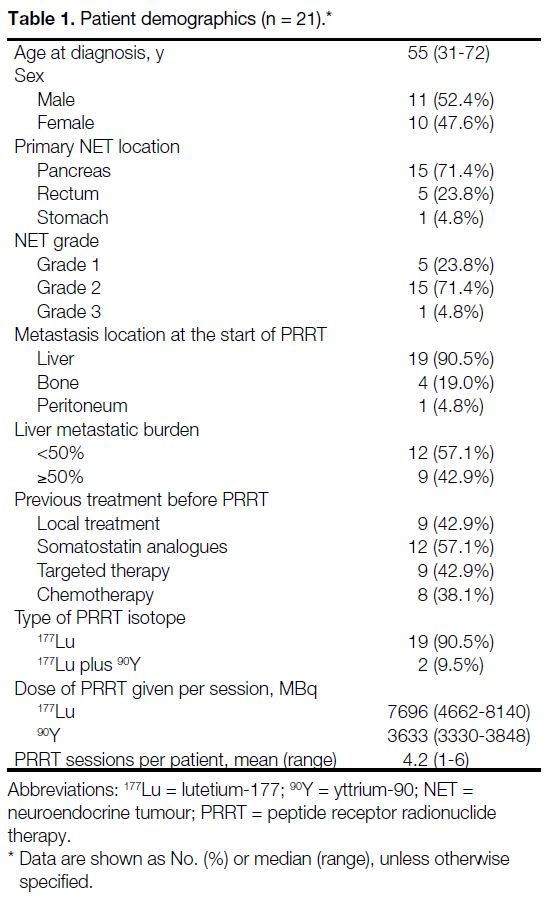
characteristics are summarised in Table 1. The median
age at diagnosis was 55 years (range, 31-72), with 11
male and 10 female patients. All patients had an Eastern
Cooperative Oncology Group performance status score of 0
to 1. The most common primary tumour site was the
pancreas (71.4%), followed by the rectum (23.8%)
and stomach (4.8%). According to World Health
Organization tumour grading, 23.8% were Grade 1,
71.4% were Grade 2, and 4.8% were Grade 3.
Liver metastases were present in 90.5% of patients,
followed by bone metastases (19.0%) and peritoneal
metastases (4.8%). About 43% of patients had a hepatic
metastatic burden of 50% or more of liver volume on
baseline imaging.
Table 1. Patient demographics (n = 21).
The median interval between diagnosis and initiation of
PRRT was 12.5 months (range, 2.5-86.2). A total of 42.9%
of patients had received prior locoregional treatment,
including surgery or transarterial chemoembolisation,
before undergoing PRRT. Overall, 85.7% of patients
received PRRT after the failure of at least one systemic
treatment, which included somatostatin analogues (n = 12), everolimus (n = 9), and chemotherapy agents such
as temozolomide plus capecitabine or oxaliplatin plus
capecitabine (n = 8). The median number of systemic
treatments before PRRT was 1 (range, 0-3). PRRT was used as first-line treatment in 14.3% of patients,
as second-line in 61.9%, and as third-line or beyond in
23.8%. No patient received additional local or systemic
treatment between PRRT cycles.
177Lu was used as monotherapy in 90.5% of patients,
while a combination of 177Lu and 90Y was used in 9.5%.
In the 177Lu alone group, patients received a median dose
of 7622 MBq per injection (range, 4662-8140). In the
combination group, the median dose was 7844 MBq of
177Lu (range, 5920-8140) and 3633 MBq of 90Y (range,
3330-3848). Patients underwent a mean of 4.2 PRRT
cycles. Five patients were unable to complete at least
four cycles: two received one cycle, two received two
cycles, and one received three cycles, due to disease
progression or death.
All patients were assessed for radiological response
using the RECIST 1.1 criteria. Among the 21 patients, partial response was observed in 47.6%, stable disease
in 23.8%, and disease progression in 28.6%. No patient
achieved a complete response. The disease control rate
(defined as the proportion of patients with either partial
response or stable disease) was 71.4%.
Progression-Free Survival
The median follow-up duration after the last PRRT
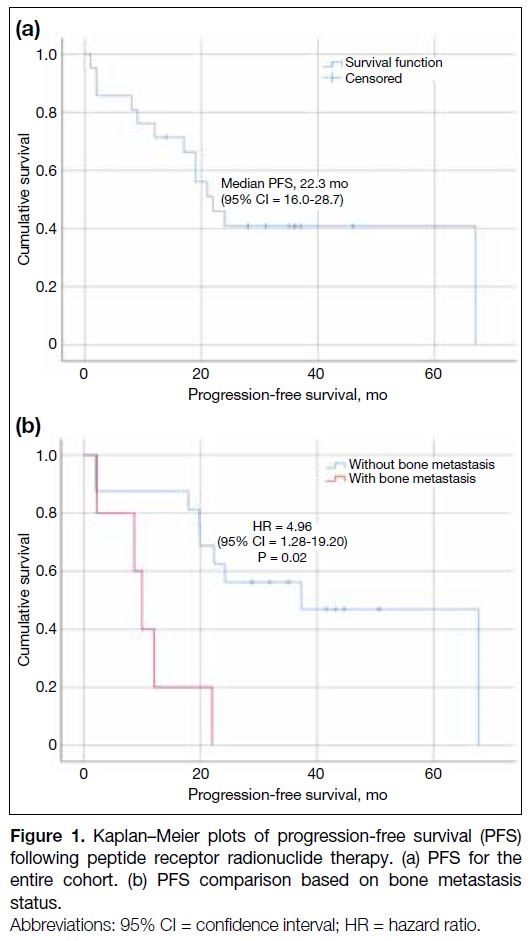
treatment was 19 months (range, 2-44). Median PFS
was estimated at 22.3 months (95% confidence interval
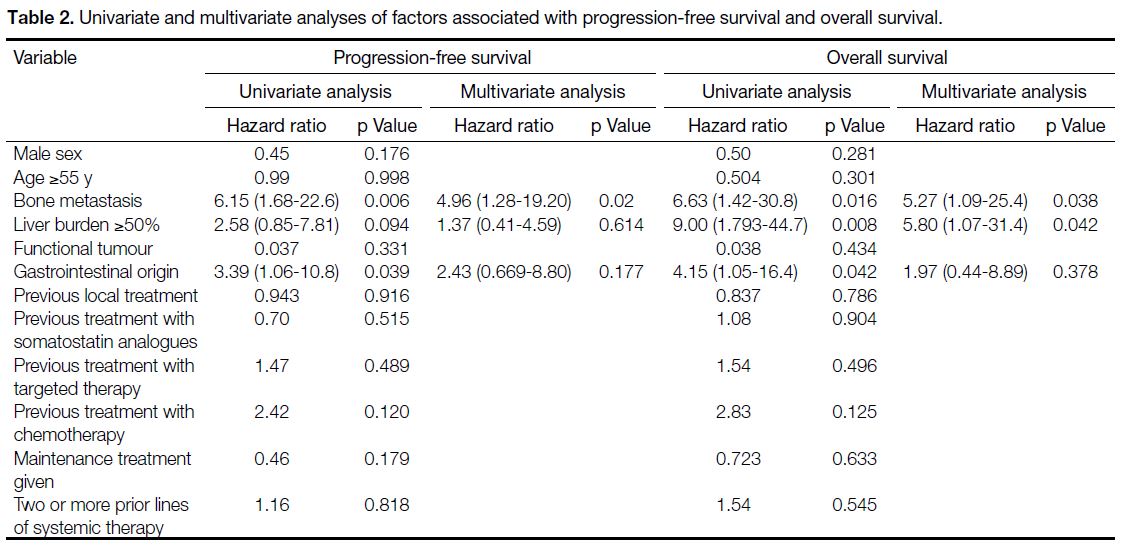
[95% CI] = 16.0-28.7) [Figure 1a]. Univariate analysis (Table 2) identified significantly shorter PFS in patients with
bone metastasis (p = 0.006) and GI primary NETs
(p = 0.039). Bone metastases remained an independent
prognostic factor in multivariate analysis, with a hazard
ratio (HR) of 4.96 (95% CI = 1.28-19.20; p = 0.02) [Figure 1b]. Age, sex, prior treatment with somatostatin
analogues, targeted therapy, chemotherapy, and
maintenance treatment were not significantly associated
with PFS.
Figure 1. Kaplan–Meier plots of progression-free survival (PFS) following peptide receptor radionuclide therapy. (a) PFS for the entire cohort. (b) PFS comparison based on bone metastasis status.
Table 2. Univariate and multivariate analyses of factors associated with progression-free survival and overall survival.
Overall Survival
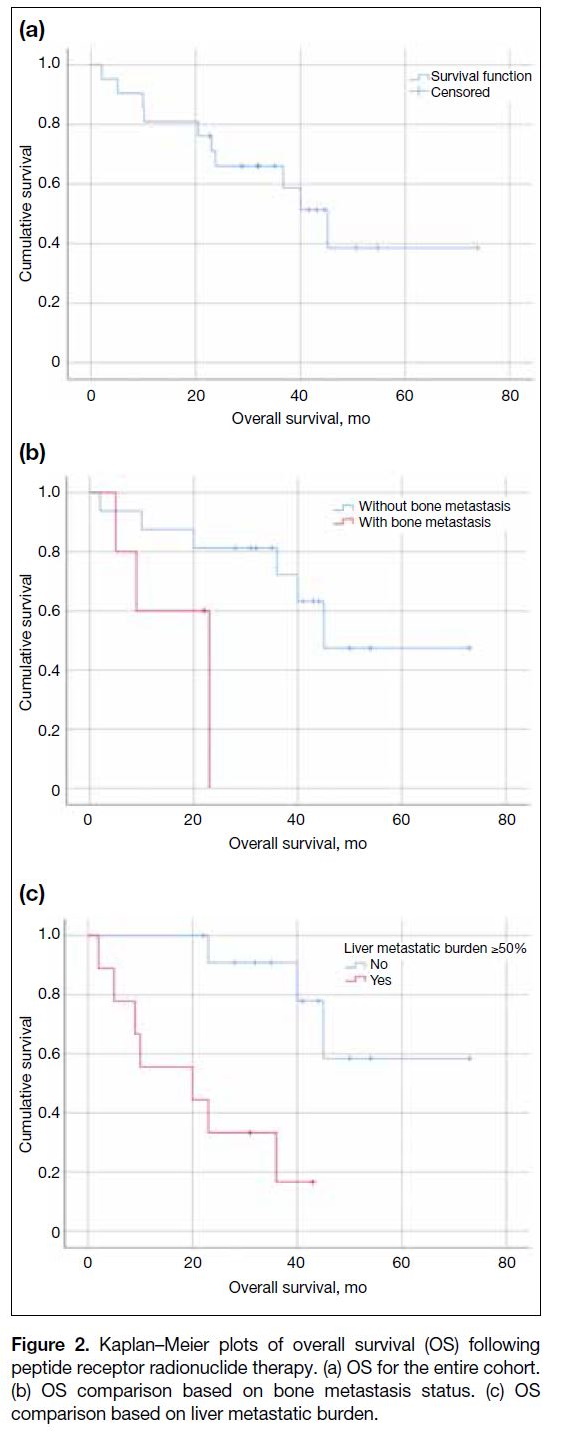
The median OS was 45.2 months (95% CI = 33.4-57.0)
[Figure 2a]. Univariate analysis of baseline factors
potentially associated with OS is shown in Table 2.
In the unadjusted analysis, significantly shorter OS
was associated with bone metastasis (p = 0.016), liver
metastatic burden of 50% or more (p = 0.008), and GI
primary tumours (p = 0.042). Following multivariate
analysis, both bone metastasis (HR = 5.27, 95% CI = 1.09-25.4; p = 0.038) and a high liver metastatic burden
of 50% or more (HR = 5.80, 95% CI = 1.07-31.4;
p = 0.042) remained independently associated with
poorer OS (Figure 2b and c).
Figure 2. Kaplan–Meier plots of overall survival (OS) following
peptide receptor radionuclide therapy. (a) OS for the entire cohort.
(b) OS comparison based on bone metastasis status. (c) OS
comparison based on liver metastatic burden.
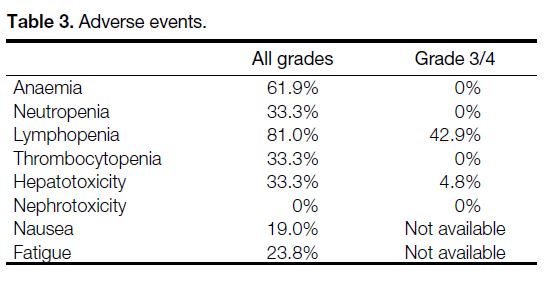
Toxicities
Patients were evaluated for haematologic, hepatic, or
renal toxicity using CTCAE version 4.03 (Table 3).
Among haematological toxicities, lymphopenia was the
only Grade 3/4 event in 42.9% of patients. No cases of
myelodysplastic syndrome were reported. Hepatotoxicity
(defined as elevation of alanine transaminase or bilirubin level)
was observed in 33.3% of patients at any grade, with
4.8% experiencing Grade 3/4 toxicity. No renal toxicity
(defined as elevated plasma creatinine level) was observed. Additionally, nausea was reported in 19.0% and fatigue
in 23.8% of patients.
Table 3. Adverse events.
Retreatment with Peptide Receptor
Radionuclide Therapy
Two patients in our cohort underwent retreatment with
PRRT after initial tumour progression. The first patient,
who achieved a PFS of 19.9 months after the initial five
cycles of 177Lu PRRT, received a further five cycles of
PRRT upon progression and achieved a partial response,
with a subsequent PFS of 20 months. The second patient
was undergoing a fourth cycle of PRRT retreatment, and
the initial post-therapy scan showed partial response. No
Grade 3/4 toxicities were observed except for Grade 3
lymphopenia in one patient.
DISCUSSION
To the best of our knowledge, this is the first local study
to examine how effective PRRT is for treating NETs in
Chinese patients from Hong Kong. An objective response
rate (ORR) of 47.6% was observed, with a median
PFS of 22.3 months, and a median OS of 45.2 months.
The OS outcome closely mirrors that reported in the
NETTER-1 trial, which included only patients with well-differentiated
midgut NETs and reported a median OS of
48 months in the 177Lu group, compared to 36.3 months
in the control group.[16] In contrast, our study included
a more heterogeneous group of primary tumours,
particularly characterised by a significant proportion of pancreatic NETs. A recent large retrospective study
by Mitjavila et al,[17] which evaluated 522 patients with
a heterogeneous group of NETs including pancreatic,
midgut, and bronchopulmonary subtypes, reported an
ORR of 33.9%, a median PFS of 24.3 months and a
median OS of 42.3 months. Our results align with these
findings, further supporting the efficacy of PRRT in
treating metastatic NETs across various primary sites in
a Chinese patient population.
Our study demonstrated that bone metastases were
significantly associated with poorer PFS and OS in
multivariate analysis. This finding aligns with the results
from Sitani et al,[18] who analysed a cohort of 468 patients
and reported that bone metastasis significantly impacted
OS. Similarly, Abou Jokh Casas et al[19] observed an
inverse relationship between OS and the presence of
bone metastases in their cohort of 36 patients treated
with PRRT for gastroenteropancreatic NETs. The
concordance of our findings with these studies supports
the role of bone metastases as a potentially negative
prognostic factor in metastatic NETs. The challenges
posed by bone metastases may be attributed to the more
aggressive nature of the disease, as bone metastases
are considered a late event in NETs[20] and predisposes
patients to serious skeletal events such as spinal cord
compression.[21]
Our study also showed that a high liver metastatic
burden of 50% or more, as assessed by radiological
evaluation, was associated with poorer OS. A visual
semi-quantitative assessment method for liver tumour
burden was used, as recommended by the European
Neuroendocrine Tumor Society,[22] and has been
shown to be reliably reproducible.[20] The presence of
neuroendocrine liver metastases is one of the most
significant negative prognostic factors for long-term survival in patients with NETs.[23] A retrospective study
by Ezziddin et al[11] analysed 68 patients with pancreatic
NETs treated with 177Lu PRRT and showed that a
liver metastatic burden greater than 25% or above was
associated with reduced OS. Our findings also align
with a recent retrospective study by Swiha et al,[24] which
demonstrated that liver metastases involving more than
50% of liver volume was associated with poorer OS.
Although there was a trend towards poorer PFS with
high liver metastatic burden, this was not statistically
significant in multivariate analysis, likely due to the
limited sample size and confounding factors.
Previous studies have suggested that tumours of GI
origin generally have a better prognosis compared to
those of pancreatic origin.[25] [26] However, our univariate
analysis showed that pancreatic origin might have
a better prognosis, though this was not significant
in the multivariate analysis, indicating the influence
of potential heterogeneity and confounding factors.
Notably, prior research has shown that GI-origin NETs
are typically associated with lower-grade tumours.[27] In
contrast, within our cohort, 83.3% of GI-origin cases
were classified as Grade 2 tumours or higher (including one
Grade 3 tumour), whereas 73% of pancreatic-origin cases
were all Grade 2 tumours and no Grade 3 tumours. Because only
patients with progressive disease were referred for
PRRT, our GI subgroup likely represents a selection of
more aggressive cases. The inclusion of more aggressive
histological subtypes within the GI-origin group in
our cohort may explain the unexpected findings in our
analysis.
Grade 3 NETs are associated with a worse prognosis
compared to their lower-grade counterparts,[25] making
treatment more challenging due to their aggressive
nature. In our study, we had only one case of Grade 3 NET
treated with PRRT, limiting further statistical analysis.
The use of PRRT in Grade 3 NETs has mainly been supported
by retrospective studies of heterogeneous groups.[12] [17] [18] [19]
However, the recent Phase 3 NETTER-2 trial
demonstrated significant improvements in PFS and ORR
with 177Lu-dotatate as a first-line treatment for higher-grade
Grade 2 and Grade 3 NETs.[28] The primary analysis showed a median PFS of 22.8 months in the 177Lu-dotatate group
compared to 8.5 months in the control group, and an
ORR of 43.0% versus 9.3%, respectively.[28] These findings
underscore the potential efficacy of PRRT in treating
high-grade NETs and suggest a promising therapeutic
option for this challenging subgroup.
The toxicity profile observed in our study was generally
favourable. The NETTER-1 study reported no Grade 3
anaemia, 1% neutropenia, and 2% thrombocytopenia.[16] Our study similarly found no Grade 3/4 anaemia or
neutropenia. However, while NETTER-1 reported 9%
Grade 3/4 lymphopenia,[16] our study observed a higher
rate (42.9%), which may be attributed to the greater
number of PRRT cycles administered. The toxicities
were reversible, and none of the patients developed
myelodysplastic syndrome. Only one patient developed
Grade 3/4 liver derangement, and none developed
any grade of renal toxicity, aligning with published
retrospective studies that showed minimal Grade 3
toxicities of liver and renal function.[13] [17]
Finally, two patients in our cohort underwent retreatment
with PRRT after tumour progression, receiving
additional distinct PRRT treatment courses. Both
patients showed a partial response. One patient is still
undergoing treatment at the time of writing, and the
other demonstrated a similar PFS between the two series
of PRRT. No significant toxicities other than Grade 3
lymphopenia were observed.
According to a meta-analysis by Strosberg et al,[29] the
median PFS following PRRT retreatment was 12.5
months, and the median OS was 26.8 months. The
study also reported a low rate of Grade 3/4 toxicities,[29] confirming that PRRT retreatment generally maintains a
manageable safety profile. It was also observed that PFS
decreased after the second treatment course compared to
the first.[30] Despite a limited number of retreatment cases
in our cohort, the results are encouraging.
Limitations
Our study has several limitations. First, as a retrospective
study, it is subject to inherent selection bias and recall
bias. Selection bias may have occurred because PRRT
is an expensive treatment, making it more accessible
to patients with better overall health and higher
socio-economic status, potentially skewing the results
towards more favourable outcomes. Recall bias may
have influenced our findings since the data relied on
medical records and follow-up reports, which might
not have consistently captured all relevant information.
Second, the sample size is relatively small and the
study population was heterogeneous, which may limit the
generalisability of the findings and introduce variability
that could confound the results. Third, the estimation
of PFS may have been affected by variability in the
timing of CT scans across different patients, potentially leading to inconsistencies and bias in assessing disease
progression. Finally, the small number of patients
undergoing PRRT retreatment limited our ability to draw
robust conclusions about the efficacy and safety of repeat
treatments.
CONCLUSION
This study provides important insights into the use of
PRRT in Chinese patients with metastatic NETs. Our
findings suggest that PRRT is an effective and generally
well-tolerated treatment option for this population,
supporting its use in local clinical practice. We identified
bone metastasis as a significant factor for worse PFS
and OS, while a high liver metastatic burden was
significantly associated with worse OS. These results can
help clinicians in Hong Kong optimise patient selection
and management strategies for the treatment of NETs.
However, larger prospective studies are needed to further
validate these findings.
REFERENCES
1. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al.
One hundred years after “carcinoid”: epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in
the United States. J Clin Oncol. 2008;26:3063-72. Crossref
2. Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring
the rising incidence of neuroendocrine tumors: a population-based
analysis of epidemiology, metastatic presentation, and outcomes.
Cancer. 2015;121:589-97. Crossref
3. Chan DT, Luk AO, So WY, Kong AP, Chow FC, Ma RC,
et al. Natural history and outcome in Chinese patients with
gastroenteropancreatic neuroendocrine tumours: a 17-year
retrospective analysis. BMC Endocr Disord. 2016;16:12. Crossref
4. Pavel M, Kidd M, Modlin I. Systemic therapeutic options for carcinoid. Semin Oncol. 2013;40:84-99. Crossref
5. Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J
Gastroenterol. 2012;47:941-60. Crossref
6. Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M,
Sedláčková E, et al. Lanreotide in metastatic enteropancreatic
neuroendocrine tumors. N Engl J Med. 2014;371:224-33. Crossref
7. Krenning EP, Kwekkeboom DJ, Oei HY, Reubi JC, van Hagen PM, Kooij PP, et al. Somatostatin receptor imaging of endocrine
gastrointestinal tumors. Schweiz Med Wochenschr. 1992;122:634-7.
8. Camus B, Cottereau AS, Palmieri LJ, Dermine S, Tenenbaum F,
Brezault C, et al. Indications of peptide receptor radionuclide therapy
(PRRT) in gastroenteropancreatic and pulmonary neuroendocrine
tumors: an updated review. J Clin Med. 2021;10:1267. Crossref
9. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine
tumors. N Engl J Med. 2017;376:125-35. Crossref
10. US Food and Drug Administration. FDA approves lutetium Lu 177 dotatate for treatment of GEP-NETS. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lutetium-lu-177-dotatate-treatment-gep-nets. Accessed
26 Nov 2024.
11. Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine
tumours. Eur J Nucl Med Mol Imaging. 2014;41:925-33. Crossref
12. Katona BW, Roccaro GA, Soulen MC, Yang YX, Bennett BJ,
Riff BP, et al. Efficacy of peptide receptor radionuclide therapy
in a United States–based cohort of metastatic neuroendocrine
tumor patients: single-institution retrospective analysis. Pancreas.
2017;46:1121-6. Crossref
13. Hamiditabar M, Ali M, Roys J, Wolin EM, OʼDorisio TM,
Ranganathan D, et al. Peptide receptor radionuclide therapy with
177Lu-octreotate in patients with somatostatin receptor expressing
neuroendocrine tumors: six years' assessment. Clin Nucl Med.
2017;42:436-43. Crossref
14. Baudin E, Walter TA, Beron A, Smith D, Hadoux J, Lachachi C, et al.
887O First multicentric randomized phase II trial investigating
the antitumor efficacy of peptide receptor radionuclide therapy
with 177Lutetium-Octreotate (OCLU) in unresectable progressive
neuroendocrine pancreatic tumor: Results of the OCLURANDOM
trial. Ann Oncol. 2022;33(Suppl 7):S954. Crossref
15. Saravana-Bawan B, Bajwa A, Paterson J, McEwan AJ,
McMullen TP. Efficacy of 177Lu peptide receptor radionuclide
therapy for the treatment of neuroendocrine tumors: a meta-analysis.
Clin Nucl Med. 2019;44:719-27. Crossref
16. Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, et al. 177Lu-Dotatate plus long-acting octreotide
versus high dose long-acting octreotide in patients with midgut
neuroendocrine tumours (NETTER-1): final overall survival and
long-term safety results from an open-label, randomised, controlled,
phase 3 trial. Lancet Oncol. 2021;22:1752-63. Crossref
17. Mitjavila M, Jimenez-Fonseca P, Belló P, Pubul V, Percovich JC,
Garcia-Burillo A, et al. Efficacy of [177Lu] Lu-DOTATATE in
metastatic neuroendocrine neoplasms of different locations:
data from the SEPTRALU study. Eur J Nucl Med Mol Imaging.
2023;50:2486-500. Crossref
18. Sitani K, Parghane RV, Talole S, Basu S. Long-term outcome of indigenous 177Lu-DOTATATE PRRT in patients with metastatic
advanced neuroendocrine tumours: a single institutional observation
in a large tertiary care setting. Br J Radiol. 2021;94:20201041. Crossref
19. Abou Jokh Casas E, Pubul Núñez V, Anido-Herranz U, Del Carmen Mallón Araujo M, Del Carmen Pombo Pasín M, Garrido Pumar
M, et al. Evaluation of 177Lu-Dotatate treatment in patients with
metastatic neuroendocrine tumors and prognostic factors. World J
Gastroenterol. 2020;26:1513-24. Crossref
20. Scopel M, De Carlo E, Bergamo F, Murgioni S, Carandina R,
Cervino AR, et al. Bone metastases from neuroendocrine
tumors: clinical and biological considerations. Endocr Connect.
2022;11:e210568. Crossref
21. Van Loon K, Zhang L, Keiser J, Carrasco C, Glass K, Ramirez MT,
et al. Bone metastases and skeletal-related events from
neuroendocrine tumors. Endocr Connect. 2015;4:9-17. Crossref
22. Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T,
et al. ENETS consensus guidelines for the management of patients
with liver and other distant metastases from neuroendocrine
neoplasms of foregut, midgut, hindgut, and unknown primary.
Neuroendocrinology. 2012;95:157-76. Crossref
23. Zappa M, Hentic O, Vullierme MP, Lagadec M, Ronot M, Ruszniewski P, et al. Is visual radiological evaluation of
liver tumour burden in patients with neuroendocrine tumours
reproducible? Endocr Connect. 2017;6:33-8. Crossref
24. Swiha MM, Sutherland DE, Sistani G, Khatami A, Abazid RM, Mujoomdar A, et al. Survival predictors of 177Lu-Dotatate peptide
receptor radionuclide therapy (PRRT) in patients with progressive
well-differentiated neuroendocrine tumors (NETS). J Cancer Res Clin Oncol. 2022;148:225-36. Crossref
25. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends
in the incidence, prevalence, and survival outcomes in patients
with neuroendocrine tumors in the United States. JAMA Oncol.
2017;3:1335-42. Crossref
26. Man D, Wu J, Shen Z, Zhu X. Prognosis of patients with neuroendocrine tumor: a SEER database analysis. Cancer Manag
Res. 2018;10:5629-38. Crossref
27. Korse CM, Taal BG, van Velthuysen ML, Visser O. Incidence and
survival of neuroendocrine tumours in the Netherlands according
to histological grade: Experience of two decades of cancer registry.
Eur J Cancer. 2013;49:1975-83. Crossref
28. Singh S, Halperin D, Myrehaug S, Herrmann K, Pavel M, Kunz PL, et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of
newly diagnosed, advanced grade 2-3, well-differentiated,
gastroenteropancreatic neuroendocrine tumours (NETTER-2): an
open-label, randomised, phase 3 study. Lancet. 2024;403:2807-17. Crossref
29. Strosberg J, Leeuwenkamp O, Siddiqui MK. Peptide receptor
radiotherapy re-treatment in patients with progressive
neuroendocrine tumors: a systematic review and meta-analysis.
Cancer Treat Rev. 2021;93:102141. Crossref
30. Zacho MD, Iversen P, Villadsen GE, Baunwall SM, Arveschoug AK, Grønbaek H, et al. Clinical efficacy of first and second series
of peptide receptor radionuclide therapy in patients with
neuroendocrine neoplasm: a cohort study. Scand J Gastroenterol.
2021;56:289-97. Crossref