Magnetic Resonance Imaging of Invasive Ductal Carcinoma and Ductal Carcinoma in Situ in Detecting Multifocal/Multicentric and Bilateral Breast Disease: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2025;28:Epub 10 September 2025
Magnetic Resonance Imaging of Invasive Ductal Carcinoma and Ductal Carcinoma in Situ in Detecting Multifocal/Multicentric and Bilateral Breast Disease: A Pictorial Essay
YY Man1, YH Chan2, HL Chan1, LC Chan1, KH Wong1, KF Tam1, HL Chau2
1 Department of Radiology, North District Hospital, Hong Kong SAR, China
2 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong SAR, China
Correspondence: Dr YY Man, Department of Radiology, North District Hospital, Hong Kong SAR, China. Email: manyan93@connect.hku.hk
Submitted: 27 July 2024; Accepted: 14 April 2025. This version may differ from the final version when published in an issue.
Contributors: YYM and HLC designed the study. YYM acquired the data. All authors analysed the data. YYM drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the New Territories East Cluster Research Ethics Committee/Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: 2024.270). Patient consent was waived by the Board due to the retrospective nature of the study.
BACKGROUND
In accordance with the standard protocol
in place, all patients with biopsy-proven
breast malignancy (either by ultrasound-guided
or stereotactic biopsy), with histology of invasive
ductal carcinoma (IDC) and/or ductal carcinoma in situ
(DCIS), subsequently undergo preoperative magnetic
resonance imaging (MRI) of both breasts to rule out
multifocal/multicentric and bilateral disease before
considering breast-conserving therapy (BCT). Some of
the patients are referred from the surgical department
to the radiology department for MRI if they do not opt
for private imaging. Unlike invasive lobular carcinoma
(ILC) which is characteristically associated with
multifocal/multicentric or bilateral disease,[1] primary
IDC and DCIS are not known to be linked to a high rate
of such involvement, and patients may proceed directly
to BCT without preoperative MRI in our locality. This
pictorial essay reviews our experience in detecting
multifocal/multicentric and bilateral disease in patients
with primary IDC and DCIS using MRI and illustrates
the associated MRI features.
MAGNETIC RESONANCE IMAGING FEATURE
Most primary breast carcinomas present as a palpable
mass. By definition, a ‘mass’ is a three-dimensional
lesion that occupies space. Mammography remains
the cornerstone of breast cancer screening and is often
the first imaging modality used. Ultrasound is useful
in characterising palpable masses, especially in dense
breast tissue, providing real-time assessment of lesion
morphology and vascularity. MRI is generally reserved
for problem-solving, preoperative staging, or screening
high-risk populations. Any enhancing lesion measuring
less than 5 mm on MRI is termed a ‘focus’, which is too
small to characterise. Evaluation of a mass is based on
its shape, margins, T1- and T2-weighted signals, and its
enhancement pattern.[2] MRI provides valuable functional
information regarding masses, including kinetic curves
and diffusion restriction, which will be discussed in
a subsequent section. MRI often reveals multifocal/multicentric and bilateral disease in IDC and DCIS that
is occult on mammography or ultrasound, commonly
presenting as non-mass enhancement (NME).
Non-Mass Enhancement
NME refers to an area of enhancement without an
associated mass and is the most common MRI finding in
multifocal/multicentric and bilateral disease.[2] [3] [4]
Various distribution patterns of NME on breast MRI
include focal, linear, ductal, segmental, regional,
multiple regions, and diffuse.[5] Focal NME is defined as
a single, small, confined area of abnormal enhancement
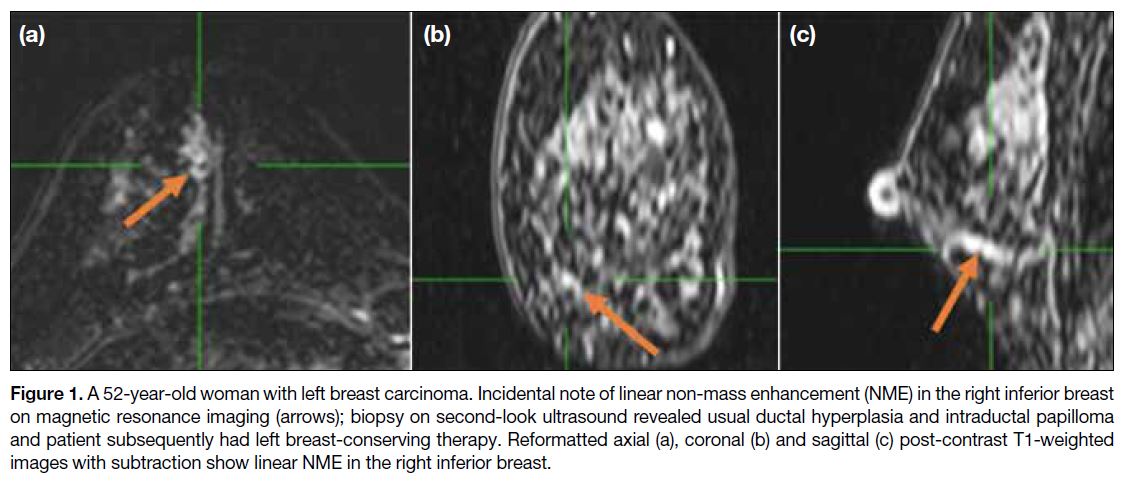
occupying less than 25% of the breast. Linear NME
appears as a line not conforming to a ductal pattern
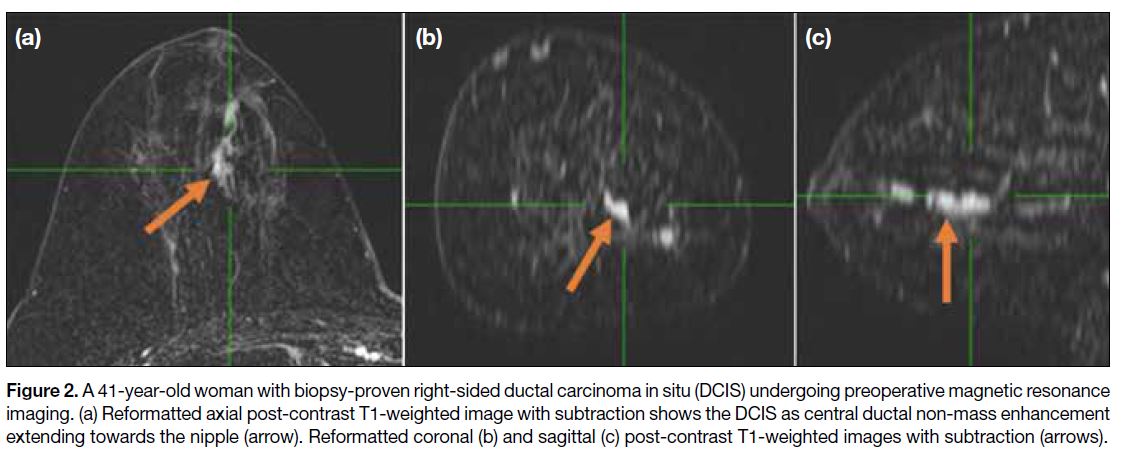
(Figure 1), while ductal NME may be linear or linear
branching corresponding to one or more ducts, usually
radiating towards the nipple (Figure 2). A mixed
pattern of linear and ductal enhancement is commonly seen. Ductal enhancement is considered suspicious for
malignancy, with a positive predictive value ranging from
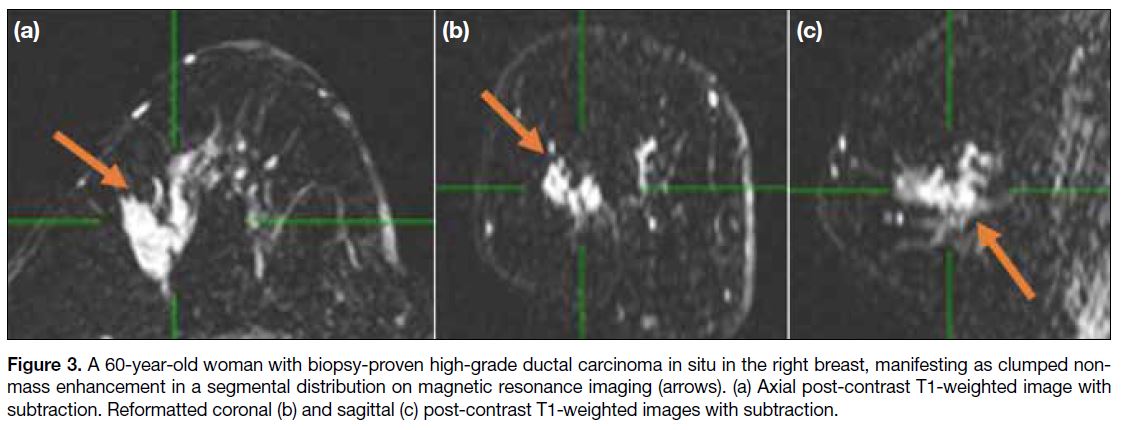
26% to 58.5%.[2] [5] Segmental enhancement (Figure 3) is
triangular or cone-shaped, representing involvement of
a single branching ductal system. Such enhancement has
a high positive predictive value for carcinoma, ranging
from 67% to 100%.[2] [6] [7] Regional enhancement involves
a larger area not conforming to a ductal distribution and
may appear geographic or patchy, potentially representing
background parenchymal enhancement or benign lesions
such as fibrocystic changes.[4] Multiple regions of NME
are defined as at least two large volumes of tissue not
conforming to ductal distribution and separated by
normal tissue or fat. Diffuse NME refers to widely
scattered, evenly distributed enhancement throughout the breast. Multiple-region and diffuse enhancement are
more characteristic of benign proliferative changes.[4]
Figure 1. A 52-year-old woman with left breast carcinoma. Incidental note of linear non-mass enhancement (NME) in the right inferior breast
on magnetic resonance imaging (arrows); biopsy on second-look ultrasound revealed usual ductal hyperplasia and intraductal papilloma
and patient subsequently had left breast-conserving therapy. Reformatted axial (a), coronal (b) and sagittal (c) post-contrast T1-weighted
images with subtraction show linear NME in the right inferior breast.
Figure 2. A 41-year-old woman with biopsy-proven right-sided ductal carcinoma in situ (DCIS) undergoing preoperative magnetic resonance
imaging. (a) Reformatted axial post-contrast T1-weighted image with subtraction shows the DCIS as central ductal non-mass enhancement
extending towards the nipple (arrow). Reformatted coronal (b) and sagittal (c) post-contrast T1-weighted images with subtraction (arrows).
Figure 3. A 60-year-old woman with biopsy-proven high-grade ductal carcinoma in situ in the right breast, manifesting as clumped non-mass
enhancement in a segmental distribution on magnetic resonance imaging (arrows). (a) Axial post-contrast T1-weighted image with
subtraction. Reformatted coronal (b) and sagittal (c) post-contrast T1-weighted images with subtraction.
The internal characteristics of NME include
homogeneous, heterogeneous, stippled/punctate,
and clumped patterns. Homogeneous enhancement
refers to confluent, uniform enhancement while
heterogeneous enhancement is non-uniform and appears
in a random pattern. Stippled/punctate enhancement
describes multiple, tiny (1-2 mm), dot-like, similar-appearing
enhancing foci that do not conform to a
ductal distribution. Clumped enhancement refers to an
aggregate of enhancing masses or foci in a cobblestone
pattern. Among non–mass-like enhancement patterns,
stippled enhancement is less likely to be malignant, with
a 25% incidence of malignancy, whereas homogenous,
heterogeneous and clumped enhancement patterns are
associated with higher likelihoods of malignancy at
67%, 53%-69% and 60%-88%, respectively.[2] [7] [8]
Kinetic Curves
Kinetic curve is derived from the time-signal intensity
curve through dynamic contrast-enhanced MRI,
reflecting the haemodynamic features of a specific
lesion. It can be interpreted in terms of early and delayed
phases. During the early phase (typically within 1-2
minutes after contrast injection), the initial rise of the
enhancement curve can be classified as slow, medium,
and rapid. An initial peak signal intensity achieved
within 90 seconds and exceeding 90% is defined as rapid
enhancement, which is highly suggestive of malignancy.
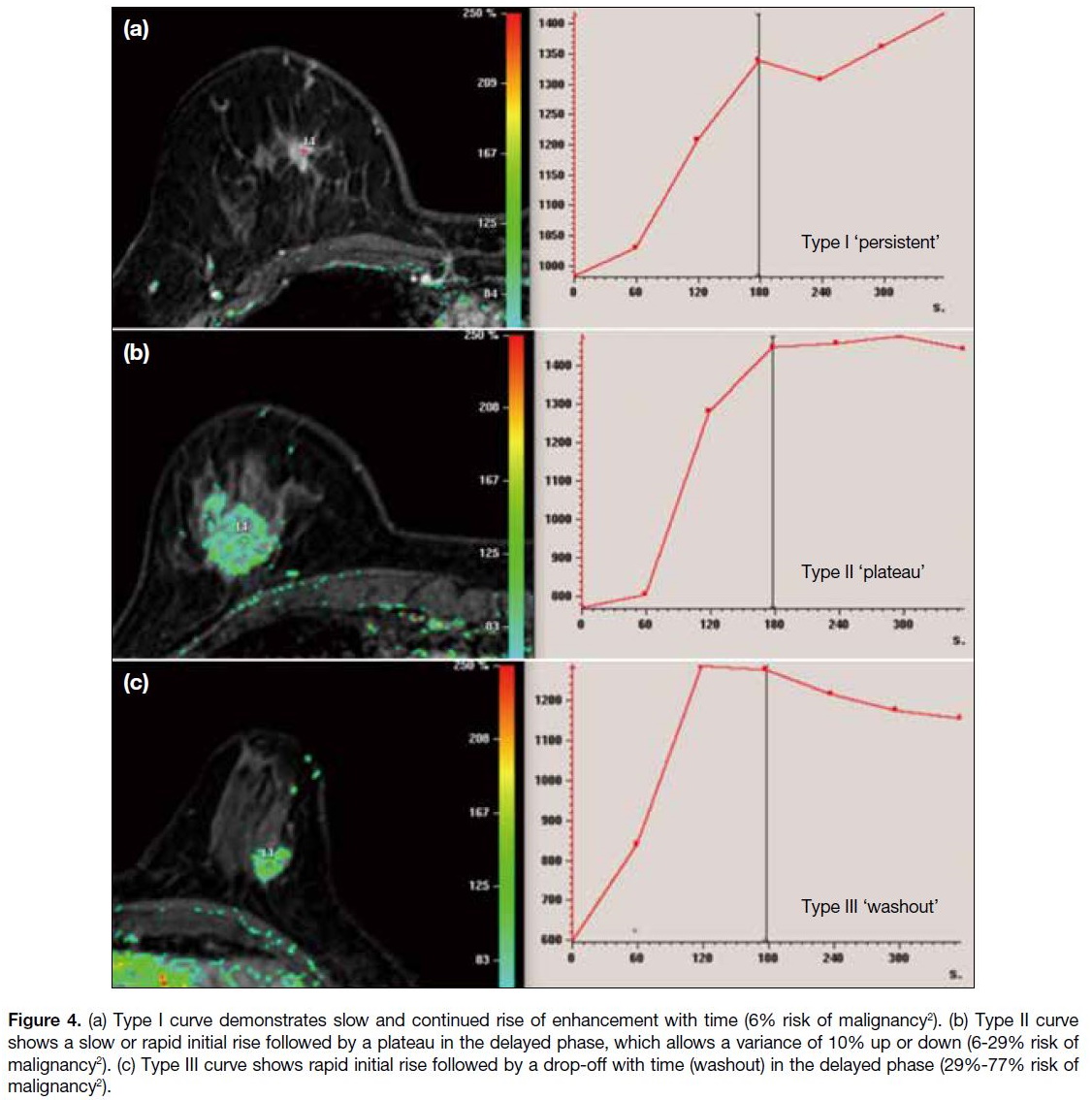
In the delayed phase (after 2 minutes), three types of
kinetic contrast enhancement are observed: persistent (type I), plateau (type II) and washout (type III).[2] These
patterns are further illustrated in Figure 4.
Figure 4. (a) Type I curve demonstrates slow and continued rise of enhancement with time (6% risk of malignancy[2]). (b) Type II curve
shows a slow or rapid initial rise followed by a plateau in the delayed phase, which allows a variance of 10% up or down (6-29% risk of
malignancy[2]). (c) Type III curve shows rapid initial rise followed by a drop-off with time (washout) in the delayed phase (29%-77% risk of
malignancy[2]).
Restricted Diffusion
The presence of restricted diffusion on diffusion-weighted
imaging indicates a higher probability of malignancy due
to increased cellularity. In equivocal cases, the apparent
diffusion coefficient (ADC) value can be measured. An
ADC value of less than 1.25 is considered to indicate
the presence of restricted diffusion, while a value of
1.25 or greater suggests its absence. The recommended
mean (± standard deviation) threshold ADC value as
1.25 ± 0.17 × 10–3 mm2/s, based on studies on the differential diagnosis of breast tumours, in which
an ADC value below this threshold indicated a malignant
lesion.[9] [10] The interpretation is illustrated in Figure 5.
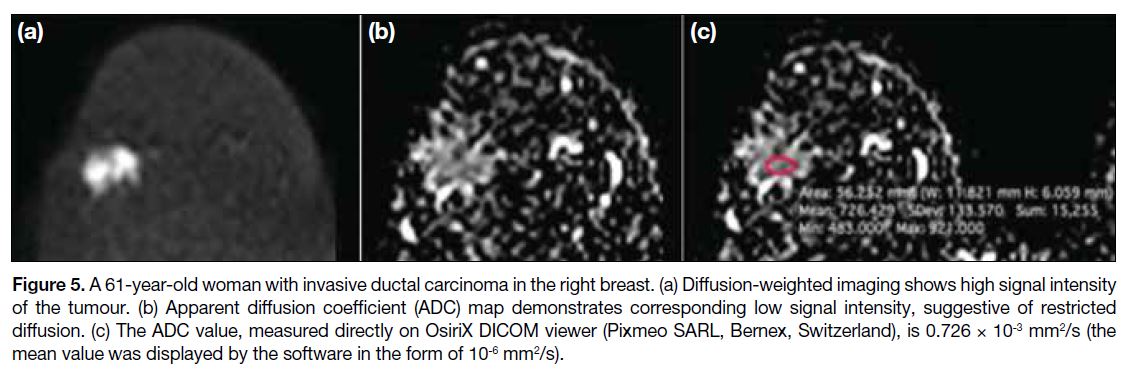
Figure 5. A 61-year-old woman with invasive ductal carcinoma in the right breast. (a) Diffusion-weighted imaging shows high signal intensity
of the tumour. (b) Apparent diffusion coefficient (ADC) map demonstrates corresponding low signal intensity, suggestive of restricted
diffusion. (c) The ADC value, measured directly on OsiriX DICOM viewer (Pixmeo SARL, Bernex, Switzerland), is 0.726 × 10-3 mm2/s (the mean value was displayed by the software in the form of 10-6 mm2/s).
OUR EXPERIENCE
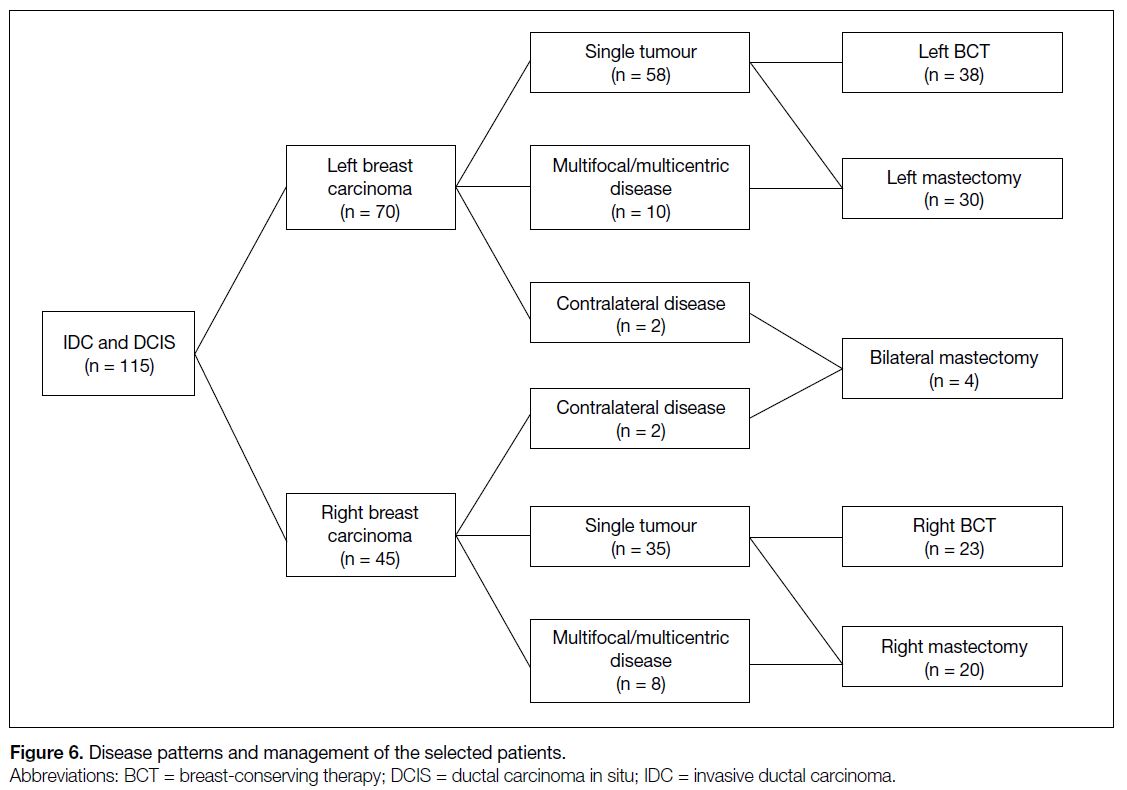
We retrospectively reviewed 115 patients with IDC
and DCIS (Figure 6). Initially, 70 patients presented
with left breast carcinoma and 45 with right breast
carcinoma. Multifocal/multicentric or bilateral disease
was identified in 22 patients, giving an incidence of
19.1%. Among those with left breast carcinoma, 10 had
ipsilateral multifocal/multicentric disease and two had
contralateral disease, thus classified as bilateral (Figure 7). Among patients with right breast carcinoma, eight
had ipsilateral multifocal/multicentric disease and two
had contralateral disease, also classified as bilateral. A
total of 61 patients underwent unilateral BCT while 54
underwent mastectomy, including four who had bilateral
mastectomy. In total, 22 patients were converted from
BCT to mastectomy. Some patients with a single ipsilateral tumour opted for mastectomy during follow-up
due to individual factors, such as fear of incomplete
excision, older age, or lack of cosmesis concern.
Figure 6. Disease patterns and management of the selected patients.
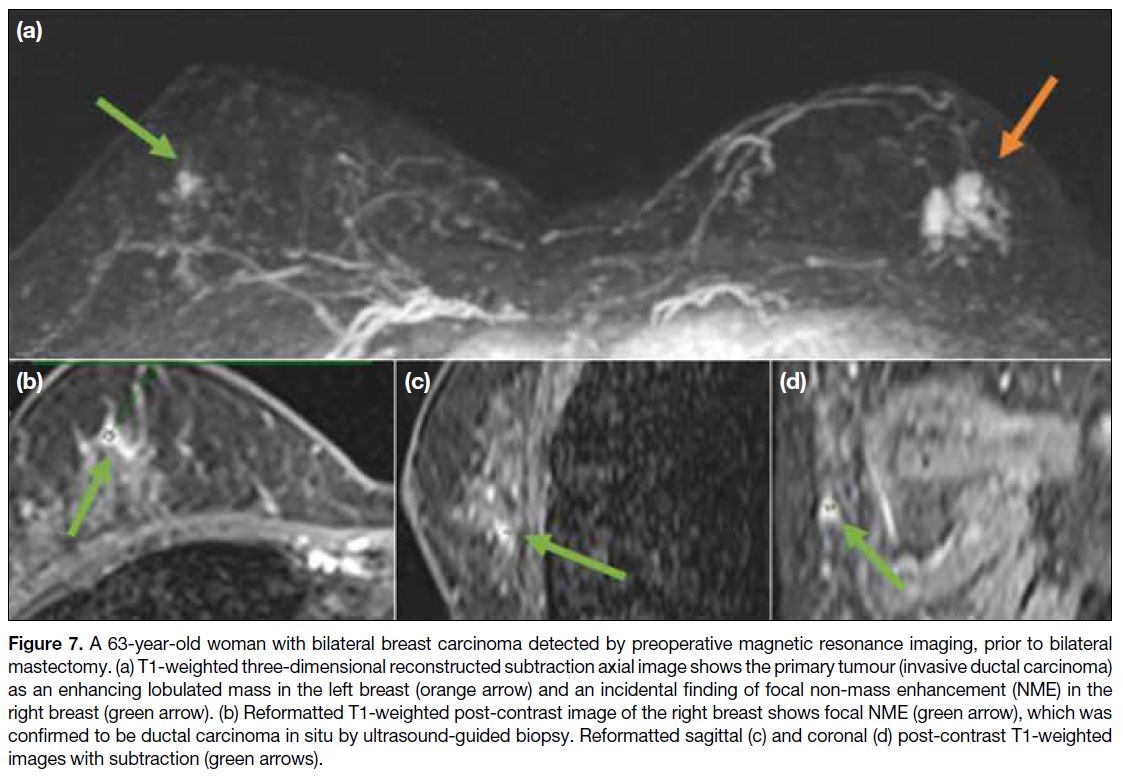
Figure 7. A 63-year-old woman with bilateral breast carcinoma detected by preoperative magnetic resonance imaging, prior to bilateral
mastectomy. (a) T1-weighted three-dimensional reconstructed subtraction axial image shows the primary tumour (invasive ductal carcinoma)
as an enhancing lobulated mass in the left breast (orange arrow) and an incidental finding of focal non-mass enhancement (NME) in the
right breast (green arrow). (b) Reformatted T1-weighted post-contrast image of the right breast shows focal NME (green arrow), which was
confirmed to be ductal carcinoma in situ by ultrasound-guided biopsy. Reformatted sagittal (c) and coronal (d) post-contrast T1-weighted
images with subtraction (green arrows).
MRI scans are reported according to the
BI-RADS (Breast Imaging Reporting and Data System)
5th Edition from the American College of Radiology.[11] The primary tumour is defined as the palpable mass or
the most suspicious lesion with biopsy-proven DCIS
or IDC, presenting as an enhancing mass or NME on
MRI. Suspicious lesions (predominantly NME) other
than the primary tumour, with a BI-RADS category
4 or higher, located in the ipsilateral or contralateral
breast, are classified as multifocal/multicentric or bilateral disease. The need for second-look
ultrasound is determined on a case-by-case basis,
influenced by patient-related factors (e.g., breast density,
family history of breast cancer) or the preferences of the
reporting radiologist and/or breast surgeon. For example,
if contralateral breast disease is identified (which greatly
affects treatment plan), or if the patient strongly desires
BCT, ultrasound is performed to guide biopsy and inform
subsequent management. If the lesion is not visible on
second-look ultrasound, particularly in cases of equivocal NME
patterns such as focal or linear distribution, MRI-guided
biopsy would be considered when clinically necessary
due to required alterations in the treatment options. If
the suspected multifocal/multicentric disease in the same
breast is deemed highly suspicious, such as clumped
areas of NME in a segmental distribution, second-look
ultrasound would not be performed, and the patient
would be advised to undergo mastectomy.
Following surgical excision, the histology report is
reviewed to assess the presence of multifocal/multicentric
and/or bilateral disease. Multifocal disease refers to foci located in the same quadrant as the primary tumour,
separated by more than 2 cm, whereas multicentric
disease indicates involvement of different quadrants
within the same breast. A background of DCIS, multiple
foci of DCIS, or IDC or DCIS in another quadrant
is defined as multifocal or multicentric disease. The
presence of DCIS or IDC in tissue specimens from both
breasts is classified as bilateral disease. Among the 115
cases, 17 were true positives, five were false negatives,
91 were true negatives and two were false positives. The
sensitivity and specificity of MRI in detecting multifocal/multicentric and bilateral disease were calculated to be
77.3% and 97.8%, respectively.
Among the five MRI false-negative cases, one of them
could be detected by mammogram, which showed
extensive grouped microcalcifications spanning more
than 3 cm and crossing two quadrants. MRI was
performed to rule out bilateral disease even though
mammogram and ultrasound were negative for the
contralateral breast, as the patient was young (37 years old at the time of diagnosis). The patient subsequently underwent mastectomy due to multicentric involvement
demonstrated in the mammogram. The remaining four
cases were negative on mammography and ultrasound, and they
eventually had mastectomy due to patient preference or
small breast size relative to the primary tumour.
In both false-positive cases, MRI showed focal NME
suspicious for multicentric involvement. Histological
diagnoses of the corresponding sites revealed atypical
apocrine adenosis (Figure 8) and fibroadenoma (Figure 9). Both patients opted for mastectomy due to previous
chest wall irradiation for contralateral breast carcinoma,
which increased the risk of toxicity with possible re-irradiation,
and because of a large tumour size that made preservation of the nipple-areolar complex impossible.
In both cases, MRI findings alone did not alter the
management.
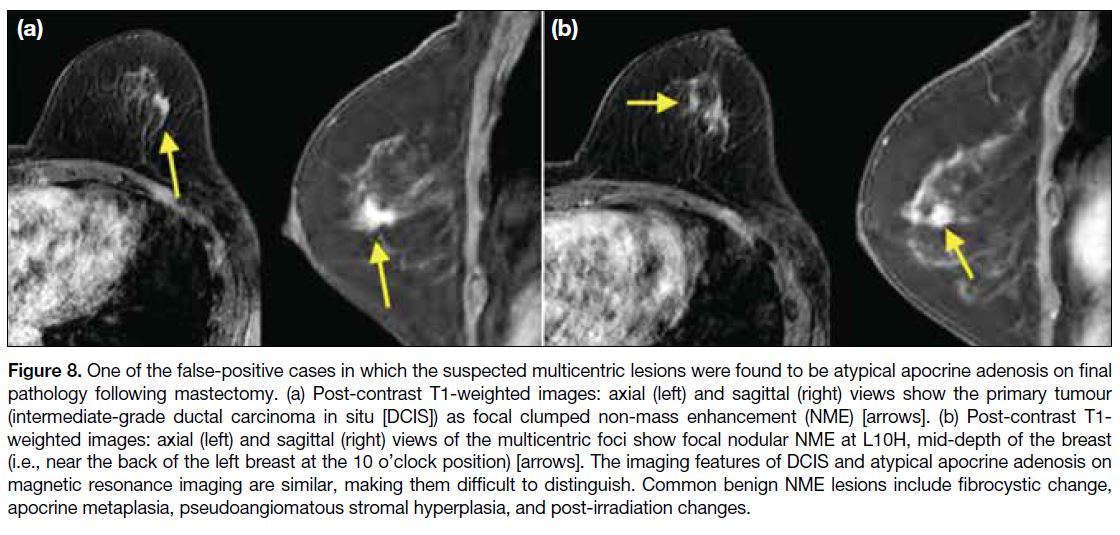
Figure 8. One of the false-positive cases in which the suspected multicentric lesions were found to be atypical apocrine adenosis on final
pathology following mastectomy. (a) Post-contrast T1-weighted images: axial (left) and sagittal (right) views show the primary tumour
(intermediate-grade ductal carcinoma in situ [DCIS]) as focal clumped non-mass enhancement (NME) [arrows]. (b) Post-contrast T1-weighted images: axial (left) and sagittal (right) views of the multicentric foci show focal nodular NME at L10H, mid-depth of the breast
(i.e., near the back of the left breast at the 10 o’clock position) [arrows]. The imaging features of DCIS and atypical apocrine adenosis on
magnetic resonance imaging are similar, making them difficult to distinguish. Common benign NME lesions include fibrocystic change,
apocrine metaplasia, pseudoangiomatous stromal hyperplasia, and post-irradiation changes.
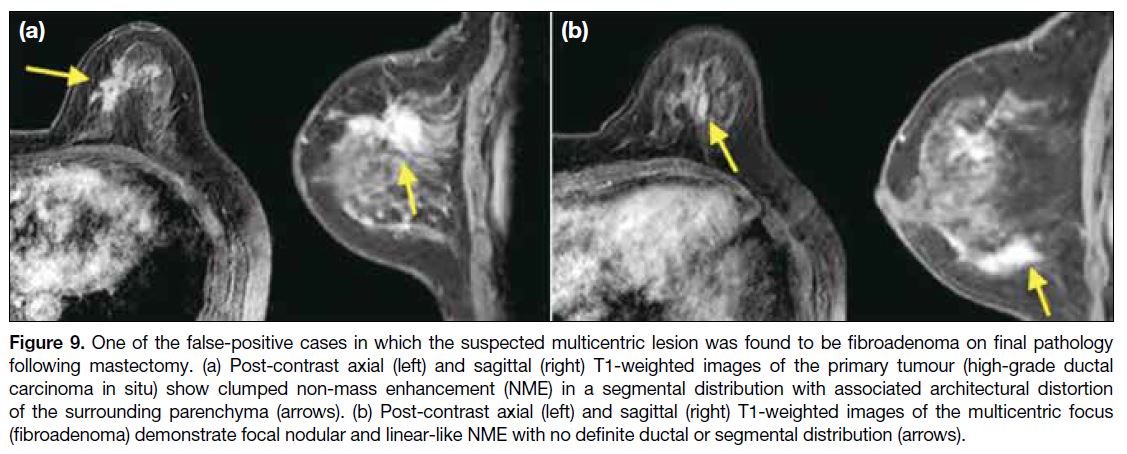
Figure 9. One of the false-positive cases in which the suspected multicentric lesion was found to be fibroadenoma on final pathology
following mastectomy. (a) Post-contrast axial (left) and sagittal (right) T1-weighted images of the primary tumour (high-grade ductal
carcinoma in situ) show clumped non-mass enhancement (NME) in a segmental distribution with associated architectural distortion
of the surrounding parenchyma (arrows). (b) Post-contrast axial (left) and sagittal (right) T1-weighted images of the multicentric focus
(fibroadenoma) demonstrate focal nodular and linear-like NME with no definite ductal or segmental distribution (arrows).
There are no well-established data in the literature
regarding the incidence of multifocal/multicentric and
bilateral disease with the histology of IDC and DCIS.
A previous study instead investigated the incidence of such
disease based on the immunohistochemical features,
including oestrogen receptor, progesterone receptor,
and human epidermal growth factor receptor 2.[12] ILC
is more frequently found to be multifocal/multicentric
or bilateral compared to DCIS and IDC, with reported
incidences commonly ranging from 10% to 20%.[13] [14] A retrospective observational study reported the incidence
of multifocal/multicentric ILC to be 18.9%,[15] which
is similar to the rate of DCIS and IDC observed in
our study. DCIS can occur independently and act as a
precursor to IDC, although the mechanism of progression
from DCIS to IDC remains poorly understood. Currently
there are no definitive imaging features that can reliably
predict which forms of DCIS are more likely to progress
to invasive cancer. The most common manifestation
of DCIS is calcification (approximately 80%), while
concomitant DCIS is found in 60% of invasive cancers
yet calcifications are only seen in 30% of those cases.
Consequently, it is not uncommon for IDC to coexist with
multifocal/multicentric DCIS, which may seem defying
to our usual knowledge about IDC. Preoperative MRI, as
the most sensitive imaging tool, plays an important role
in patients with DCIS or IDC who are planning BCT,
to rule out multifocal/multicentric disease.[16] [17] Some
lesions may be pure IDC or DCIS while others may
be DCIS progressing to IDC. The heterogeneity of this
disease thus does not exhibit any unifying or statistically
significant MRI feature. Further study regarding the
association of the immunohistochemical profile of the
tumour with its likelihood of multifocal/multicentric and
bilateral disease may be worthwhile.
Among the five false-negative cases, all involved
multifocal and multicentric low-to-intermediate-grade
DCIS in the same breast, which is known to be less readily
detected by MRI. The sensitivity of MRI for detecting
low-grade DCIS is 74.0%, and 84.1% for intermediate-grade
DCIS,[18] figures that are comparable to our study.
While DCIS most commonly presents as NME on MRI,
its detection may still be challenging in some cases. As
all patients initially underwent mammography, which
remains the gold standard for detecting calcifications,
a common feature of DCIS, the suboptimal sensitivity
of MRI in identifying low-to-intermediate-grade DCIS
could be mitigated by the complementary conventional
mammography. In our study, one case of multicentric
DCIS was detected by mammography but not by MRI,
highlighting the crucial and complementary role of
mammography in comprehensive assessment of disease
extent.[19] [20] [21] [22] [23] [24]
CONCLUSION
Based on our experience, there is a considerable
incidence of multifocal/multicentric and bilateral disease
in IDC and DCIS, for which MRI is an effective tool
for preoperative evaluation. With better knowledge of
the associated MRI features, multifocal/multicentric and bilateral disease may be more readily detected, enabling
appropriate subsequent patient management.
REFERENCES
1. Batra H, Mouabbi JA, Ding Q, Sahin AA, Raso MG. Lobular
carcinoma of the breast: a comprehensive review with translational
insights. Cancers (Basel). 2023;15:5491. Crossref
2. Schnall MD, Blume J, Bluemke DA, DeAngelis GA, DeBruhl N,
Harms S, et al. Diagnostic architectural and dynamic features at
breast MR imaging: multicenter study. Radiology. 2006;238:42-53. Crossref
3. Van Goethem M, Schelfout K, Kersschot E, Colpaert C, Weyler J,
Verslegers I, et al. Comparison of MRI features of different
grades of DCIS and invasive carcinoma of the breast. JBR-BTR.
2005;88:225-32. Crossref
4. Agrawal G, Su MY, Nalcioglu O, Feig SA, Chen JH. Significance
of breast lesion descriptors in the ACR BI-RADS MRI lexicon.
Cancer. 2009;115:1363-80. Crossref
5. Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK.
Ductal enhancement on MR imaging of the breast. AJR Am J
Roentgenol. 2003;181:519-25. Crossref
6. Liberman L, Morris EA, Lee MJ, Kaplan JB, LaTrenta LR,
Menell JH, et al. Breast lesions detected on MR imaging:
features and positive predictive value. AJR Am J Roentgenol.
2002;179:171-8. Crossref
7. Tozaki M, Igarashi T, Fukuda K. Breast MRI using the VIBE
sequence: clustered ring enhancement in the differential diagnosis
of lesions showing non-masslike enhancement. AJR Am J
Roentgenol. 2006;187:313-21. Crossref
8. Tozaki M, Fukuda K. High-spatial-resolution MRI of non-masslike
breast lesions: interpretation model based on BI-RADS MRI
descriptors. AJR Am J Roentgenol. 2006;187:330-7. Crossref
9. Dkhar W, Kadavigere R, Sukumar S, Pradhan A, Sharath S.
Diagnostic performances of ADC value in diffusion-weighted
MR imaging for differential diagnosis of breast lesions in 1.5 T: a
systematic review and meta-analysis. J Med Biol Eng. 2023;43:497-507. Crossref
10. Ng WK, Wong CK, Fung EP, Wong CW, Mak WS, Kwok KM,
et al. Association between apparent diffusion coefficient values on
diffusion weighted imaging and prognostic factors of breast cancer.
Hong Kong J Radiol. 2019;22:98-106. Crossref
11. D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS
Atlas, Breast Imaging Reporting and Data System. Reston, VA:
American College of Radiology; 2013.
12. Ilić IR, Petrović A, Živković VV, Randjelović PJ, Stojanović NM,
Radulović NS, et al. Immunohistochemical features of multifocal
and multicentric lobular breast carcinoma. Adv Med Sci.
2017;62:78-82. Crossref
13. Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM,
Dixon JM, et al. Accuracy and surgical impact of magnetic
resonance imaging in breast cancer staging: systematic review and
meta-analysis in detection of multifocal and multicentric cancer. J
Clin Oncol. 2008;26:3248-58. Crossref
14. Wilson N, Ironside A, Diana A, Oikonomidou O. Lobular breast
cancer: a review. Front Oncol. 2021;10:591399. Crossref
15. Baur A, Bahrs SD, Speck S, Wietek BM, Krämer B, Vogel U,
et al. Breast MRI of pure ductal carcinoma in situ: sensitivity of
diagnosis and influence of lesion characteristics. Eur J Radiol.
2013;82:1731-7. Crossref
16. Kuhl CK, Strobel K, Bieling H, Wardelmann E, Kuhn W, Maass N,
et al. Impact of preoperative breast MR imaging and MR-guided
surgery on diagnosis and surgical outcome of women with invasive breast cancer with and without DCIS component. Radiology.
2017;284:645-55. Crossref
17. Wang J, Li B, Luo M, Huang J, Zhang K, Zheng S, et al. Progression
from ductal carcinoma in situ to invasive breast cancer: molecular
features and clinical significance. Signal Transduct Target Ther.
2024;9:83. Crossref
18. Tajima CC, de Sousa LL, Venys GL, Guatelli CS, Bitencourt AG,
Marques EF. Magnetic resonance imaging of the breast: role in the
evaluation of ductal carcinoma in situ. Radiol Bras. 2019;52:43-7. Crossref
19. Chou SS, Romanoff J, Lehman CD, Khan SA, Carlos R, Badve SS,
et al. Preoperative breast MRI for newly diagnosed ductal carcinoma
in situ: imaging features and performance in a multicenter setting
(ECOG-ACRIN E4112 Trial). Radiology. 2021;301:66-77. Crossref
20. Lam DL, Smith J, Partridge SC, Kim A, Javid SH, Hippe DS, et al. The impact of preoperative breast MRI on surgical management
of women with newly diagnosed ductal carcinoma in situ. Acad Radiol. 2020;27:478-86. Crossref
21. Bozzini A, Renne G, Meneghetti L, Bandi G, Santos G, Vento AR,
et al. Sensitivity of imaging for multifocal-multicentric breast
carcinoma. BMC Cancer. 2008;8:275. Crossref
22. Shimauchi A, Jansen SA, Abe H, Jaskowiak N, Schmidt RA,
Newstead GM. Breast cancers not detected at MRI: review of
false-negative lesions. AJR Am J Roentgenol. 2010;194:1674-9. Crossref
23. Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M,
Lewis RS, et al. Diagnostic accuracy of mammography, clinical
examination, US, and MR imaging in preoperative assessment of
breast cancer. Radiology. 2004;233:830-49. Crossref
24. Sardanelli F, Giuseppetti GM, Panizza P, Bazzocchi M, Fausto A,
Simonetti G, et al. Sensitivity of MRI versus mammography for
detecting foci of multifocal, multicentric breast cancer in fatty and
dense breasts using the whole-breast pathologic examination as a
gold standard. AJR Am J Roentgenol. 2004;183:1149-57. Crossref