Clinical Features and Prognostic Factors in Non–Small-Cell Lung Cancer Patients Receiving Whole Brain Radiotherapy
ORIGINAL ARTICLE
Hong Kong J Radiol 2025;28:Epub 12 September 2025
Clinical Features and Prognostic Factors in Non–small-cell Lung Cancer Patients Receiving Whole Brain Radiotherapy
CY Wong, WWY Tin, WH Mui, SF Nyaw, FCS Wong
Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong SAR, China
Correspondence: Dr CY Wong, Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong SAR, China. Email: federicawong@ha.org.hk
Submitted: 2 August 2024; Accepted: 13 December 2024. This version may differ from the final version when published in an issue.
Contributors: CYW, WWYT, WHM and SFN designed the study. CYW acquired the data. CYW, WWYT, WHM and SFN analysed the data.
CYW drafted the manuscript. WWYT, WHM, SFN and FCSW critically revised the manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and
integrity.
Conflicts of Interest: As an editor of the journal, FCSW was not involved in the peer review process. Other authors have disclosed no conflicts of
interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-121-1). A waiver of patient consent was granted by the Board due to the retrospective nature of the research.
Abstract
Objective
Brain metastases are common in non–small-cell lung cancer (NSCLC) and significantly impact quality of
life and survival. Despite advances in systemic treatment and stereotactic radiotherapy, whole brain radiotherapy
(WBRT) remains frequently used during the disease course. However, prognostic tools to guide WBRT decisions are
lacking. This study aimed to identify prognostic factors in NSCLC patients with brain metastases receiving WBRT.
Methods
We conducted a retrospective study of NSCLC patients with brain metastases treated with WBRT at our hospital between January 2020 and April 2023. Overall survival (OS) was estimated using the Kaplan–Meier method. Prognostic factors for OS were identified using a multivariable Cox regression model.
Results
A total of 135 patients were included. The median OS was 138 days (95% confidence interval = 102.3-173.7). The 30-day mortality rate was 16.3% and the 1-year OS rate was 19.3%. Multivariable analysis identified a
Karnofsky Performance Scale score of 70 or above, neutrophil-to-lymphocyte ratio of smaller than 4, and systemic treatment after WBRT as independent favourable prognostic factors for OS.
Conclusion
WBRT remains an effective treatment for selected NSCLC patients with brain metastases. Karnofsky Performance Scale score of 70 or above, neutrophil-to-lymphocyte ratio of smaller than 4, and receipt of systemic treatment after WBRT were significant predictors of improved survival. Prospective studies are needed to further evaluate the role and timing of WBRT and to develop an accurate prognostic index to guide treatment decisions between WBRT and supportive care.
Key Words: Brain; Brain neoplasms; Carcinoma, non–small-cell lung; Prognosis
中文摘要
接受全腦放療的非小細胞肺癌患者的臨床特徵和預後因素
黃仲昕、佃穎恩、梅永豪、饒仕鋒、黃志成
目的
非小細胞肺癌患者常見腦轉移,對生活質素及存活率有重大影響。雖然全身治療及立體定位放療已大有進展,但全腦放療在病程中仍經常被採用。然而,目前缺乏針對全腦放療治療決策的預後工具。本研究旨在找出接受全腦放療的非小細胞肺癌腦轉移患者的預後因素。
方法
我們對2020年1月至2023年4月期間在本院接受全腦放療治療的非小細胞肺癌腦轉移患者的臨床資料進行回顧性分析。整體存活期以Kaplan–Meier方法估算,並以多變項Cox回歸模型分析預測整體存活期的相關預後因素。
結果
本研究共納入135名患者。整體中位存活期為138天(95%置信區間:102.3-173.7 天)。30天內死亡率為16.3%,一年整體存活率為19.3%。多變項分析顯示,Karnofsky表現評分(KPS)70或以上、嗜中性白血球與淋巴球比率(NLR)少於4,以及接受全腦放療後的全身治療均為獨立的有利預後因素。
結論
對於部分合適的非小細胞肺癌腦轉移患者而言,全腦放療仍是有效的治療選擇。KPS 70或以上、NLR少於4,以及全腦放療後接受全身治療與較佳存活結果顯著相關。未來應進行前瞻性研究,進一步探討全腦放療的角色與時機,並研發準確的預後評估工具,以協助臨床上在全腦放療與支持治療之間作出合適選擇。
INTRODUCTION
Brain metastases adversely affect the quality of life and survival of cancer patients. Non–small-cell lung cancer
(NSCLC) has a brain metastasis incidence of up to 40%
during its clinical course, and it is increasing due to
advances in systemic treatment and imaging.[1] [2] [3] [4] [5]
Life expectancy with steroids alone is typically 1 to 2
months; whole brain radiotherapy (WBRT) increases
this to approximately 5 months and improves symptoms
in 40% to 60% of patients.[6] [7] [8] [9] [10] [11] [12] However, the treatment
landscape for brain metastasis in NSCLC is evolving,
with WBRT now mainly reserved for patients unsuitable
for stereotactic radiosurgery/radiotherapy (SRS/SRT).
Despite concerns about the neurocognitive toxicity
of WBRT and controversy of additional survival and
quality-of-life benefits, it is still widely used and reported
as the primary treatment for brain metastases in 23.6% to
25.2% of patients in recent studies.[13] [14] [15]
The QUARTZ (Quality of Life after Treatment for
Brain Metastases) study[16] found that routine WBRT
in NSCLC patients did not improve survival or quality
of life compared with best supportive care, supporting
its omission to avoid unnecessary treatment burden and
toxicity. However, details on systemic treatment were not reported, limiting its application in the modern era,
where molecular characteristics markedly influence
NSCLC treatment.
Patient selection is critical and decisions regarding
WBRT should be personalised. Evidence remains
limited in the context of evolving systemic treatments
and other local therapies, and prognostic factors are not
consistently defined. This study aimed to review survival
outcomes and clinical characteristics in NSCLC patients
who received WBRT in our hospital.
METHODS
The study included NSCLC patients who received
WBRT at Tuen Mun Hospital, Hong Kong from 1 January 2020 to
30 April 2023. WBRT was considered for patients not
eligible for neurosurgery or SRS/SRT. Demographic
data, disease characteristics, and treatment outcomes
were retrieved from electronic medical records.
Performance status was assessed using the Karnofsky
Performance Scale (KPS) at the radiotherapy planning
clinic. Overall survival (OS) was defined as the time
from the first day of WBRT to death or when censored
(data cut-off: 12 May 2024). Statistical analysis was
performed using SPSS version 26.0 (IBM Corp, Armonk [NY], United States).
Categorical variables were summarised as frequencies
and percentages; continuous variables as medians with
interquartile ranges (IQRs). OS was estimated using the Kaplan–Meier method and compared using the log-rank test. A
multivariable Cox regression model identified prognostic
factors for OS.
In addition to clinical features included in the Disease-Specific Graded Prognostic Assessment (DS-GPA),
recursive partitioning analysis, and lung-molecular
GPA score, liver metastases, neutrophil-to-lymphocyte
ratio (NLR) of 4 or above, lymphocyte percentage, low
albumin level, and elevated albumin-to-globulin ratio have
been reported as prognostic indicators in NSCLC.[16] [17] [18] [19] [20] [21]
We investigated their prognostic value in our cohort.
RESULTS
Patient Characteristics
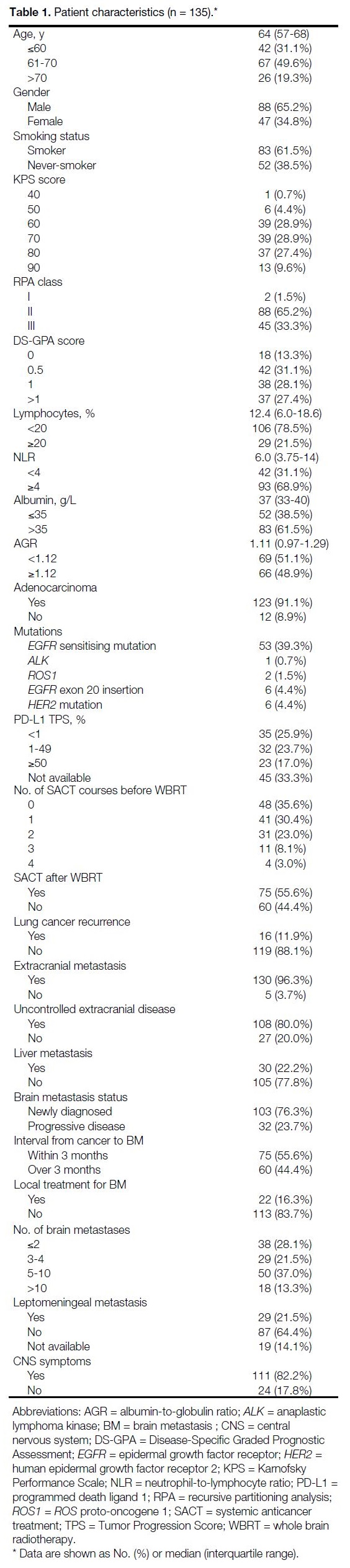
A total of 135 NSCLC patients received WBRT (median
age: 64 years; 65.2% male). Among them, 11.9%
had disease recurrence post-treatment, including one
who received chemoradiotherapy (CRT) while others
underwent surgery. The median time from surgery/CRT
to recurrence was 419 days.
Overall, 55.6% were diagnosed with brain metastases
within 3 months since the diagnosis of advanced lung
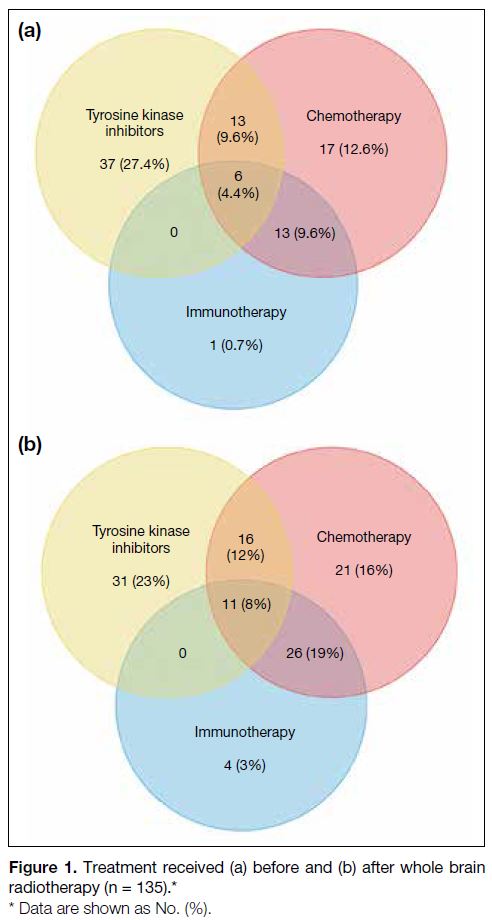
cancer. 87 patients (64.4%) and 109 patients (80.7%)
received systemic treatment before and after WBRT,
respectively (Figure 1). As shown in Table 1, 34.1%
of patients had received at least two lines of systemic
anticancer treatment. Systemic treatment post-WBRT
was given to 55.6% of patients.
Figure 1. Treatment received (a) before and (b) after whole brain
radiotherapy (n = 135).
Table 1. Patient characteristics (n = 135).
Among patients, 61.5% had a smoking history, and
91.1% had adenocarcinoma. Half (50.4%) had treatable
mutations (epidermal growth factor receptor [EGFR],
ALK [anaplastic lymphoma kinase], ROS1 [ROS proto-oncogene 1], and HER2 [human epidermal growth factor
receptor 2]). A total of 43.0% received targeted therapy
before or after WBRT; 3% received antibody-drug
conjugates.
76.3% received WBRT for newly diagnosed brain
metastasis; the rest received it upon intracranial
progression. Local treatments (surgery or SRS/SRT)
were given in 16.3%. Leptomeningeal metastasis was
present in 21.5%. All received short-course radiotherapy: four
patients receiving 30 Gy in 10 fractions and others receiving 20 Gy in five fractions. Further details are shown in Table 1.
Prognostic Factors for Survival
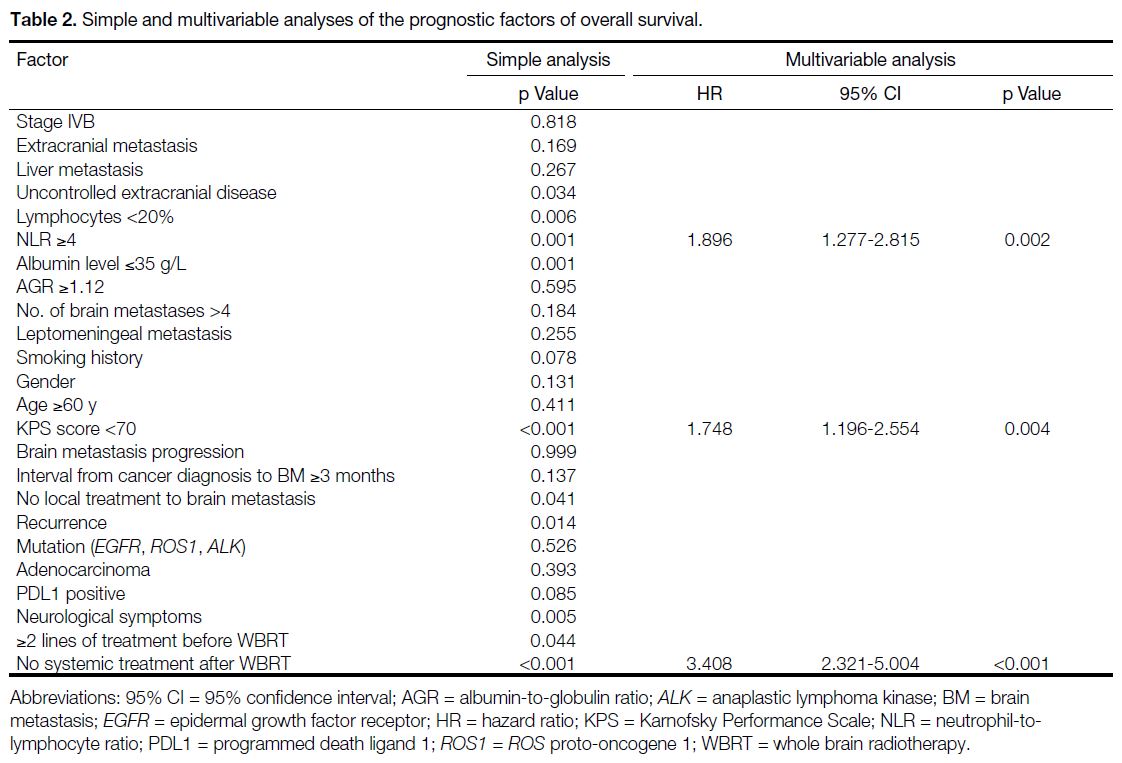
On univariate Cox regression, the following were
significant prognostic factors: KPS score of less than
70, uncontrolled extracranial disease, lymphocytes less
than 20%, NLR of 4 or above, local treatment to brain
metastasis, disease recurrence, at least two lines of
systemic treatment before WBRT, no systemic treatment
after WBRT, and presence of neurological symptoms.
Multivariable analysis identified KPS score of lower than 70, no systemic treatment after WBRT, and NLR of 4 or
above as independent poor prognostic factors (Table 2).
Table 2. Simple and multivariable analyses of the prognostic factors of overall survival.
Survival Outcomes
The median OS was 138 days (95% confidence interval
[95% CI] = 102.3-173.7). Four patients (3.0%) did not
complete WBRT due to a change in clinical condition,
all had KPS score of lower than 70. The 30-day mortality
rate was 16.3% and the 1-year OS rate was 19.3%.
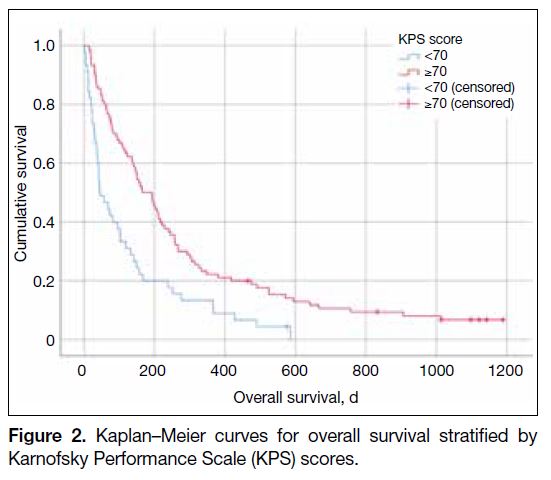
Patients with KPS score of 70 or above had significantly
better median survival than those with KPS score of
lower than 70: 165 days (95% CI = 102.3-173.7) versus
45 days (95% CI = 15.4-75.8; p < 0.001) [Figure 2].
Their 30-day mortality rates were 9.0% and 30.4%, respectively (p < 0.002). Among patients with KPS score
of 70 or above, the 1-year OS was 23.6%.
Figure 2. Kaplan–Meier curves for overall survival stratified by
Karnofsky Performance Scale (KPS) scores.
Six patients survived at data cut-off, with a median
follow-up of 1110.5 days. Three received osimertinib, two received pembrolizumab-pemetrexed-carboplatin,
and one was under surveillance after WBRT as there
was no extracranial disease progression after thoracic
chemoradiation; adjuvant durvalumab was stopped after
neurosurgery and WBRT.
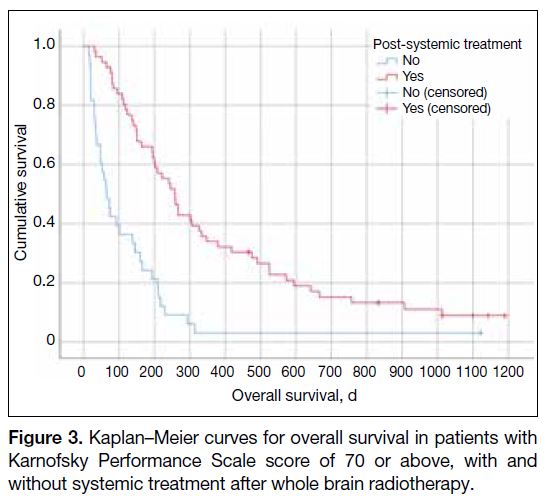
Among patients with KPS score of 70 or above, those
receiving systemic treatment post-WBRT (n = 56) had
a median survival of 257 days (95% CI = 208.8-305.2),
compared to 65 days (95% CI = 40.2-89.8) in those
without (n = 33; p < 0.001) [Figure 3].
Figure 3. Kaplan–Meier curves for overall survival in patients with
Karnofsky Performance Scale score of 70 or above, with and
without systemic treatment after whole brain radiotherapy.
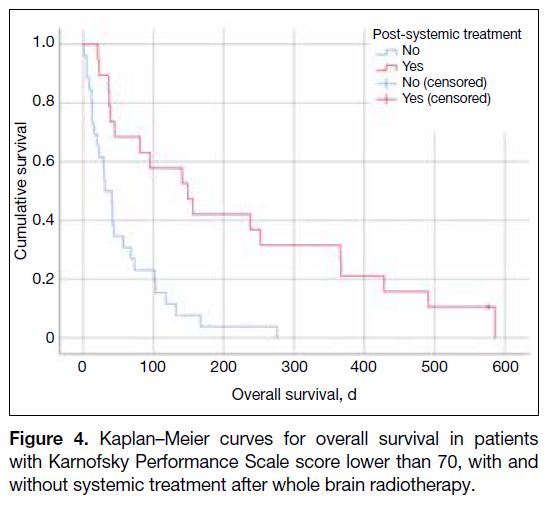
Among patients with KPS score of lower than 70,
those receiving systemic therapy post-WBRT (n = 19)
had a median survival of 149 days (95% CI = 62.3-235.7), compared to 41 days (95% CI = 25.7-56.3) in
those without (n = 27; p < 0.001) [Figure 4]. Of these
19 patients, five had not received any prior systemic
treatment. Ten patients had sensitising EGFR mutations
and were treated with erlotinib or osimertinib. Two
other patients received tyrosine kinase inhibitors, three
received chemoimmunotherapy, and two patients each
received immunotherapy alone or chemotherapy alone.
Figure 4. Kaplan–Meier curves for overall survival in patients
with Karnofsky Performance Scale score lower than 70, with and
without systemic treatment after whole brain radiotherapy.
DISCUSSION
Sensitising EGFR mutations are common in NSCLC,
present in up to 47.5% of patients according to the
2021 Hong Kong Cancer Registry data.[22] While these
mutations are linked to better survival, they are not
significantly prognostic after WBRT in our study.[23]
This may be due to the heavily pretreated nature of
these patients: among 56 with EGFG, ALK, or ROS1
mutations, 94.7% had prior systemic treatment, 47.4%
had received at least two lines of treatment, and 33.9%
had not received systemic therapy after WBRT. The
median OS from the start of WBRT was 144 days
(IQR, 9-1190), and from the time of brain metastases diagnosis
was 375 days (IQR, 25-1588).
Most prognostic tools (e.g., DS-GPA) estimate survival
from initial brain metastases diagnosis. However,
these are less applicable to patients with intracranial
progression considering WBRT (23.7% in this study),
for whom the additional prognostic factors (NLR,
systemic treatment after WBRT) identified, may be
more relevant.
Although extracranial disease is a known prognostic
factor, it was not significant in this study, likely due to the
small number of patients (3.7%) without such disease.
Age, another common factor, was also not significant for
unclear reasons.
There are no consistent guidelines for WBRT in this
population. Our findings support that good performance
status, systemic treatment after WBRT, and NLR of
smaller than 4 are associated with better survival,
aligning with recursive partitioning analysis and DS-GPA
recommendations.
Given the inconsistency in estimating post-WBRT
treatment eligibility, our analysis focused on whether
systemic treatment was administered. The median OS
was 138 days (19.7 weeks), but among patients with
poor performance status receiving only supportive care,
it was just 41 days, suggesting WBRT may be omitted
in such cases.
NLR of 4 or above was an independent prognostic
factor, reflecting increased neutrophil count and/or relative lymphopenia, a pro-inflammatory tumour
microenvironment.[19]
Prospective trials are needed to evaluate the role and
timing of WBRT in the era of evolving systemic treatment
and local therapies to develop an accurate prognostic
index to aid treatment decisions. We recommend cautious
use of WBRT, particularly in patients with KPS score of
lower than 70, no post-WBRT systemic treatment, and
NLR of 4 or above.
Limitations
The major limitation of our study is its retrospective
design, which may be related to selection bias. Patients
who did not receive WBRT were excluded. Lung
cancer is molecularly heterogeneous, yet programmed
death ligand 1 status was unavailable in one-third of
our patients. WBRT-related toxicities and quality of life
were not assessed.
CONCLUSION
WBRT remains a potentially effective treatment for
selected NSCLC patients. KPS score of 70 of above,
systemic treatment after WBRT, and NLR of smaller
than 4 were significant prognostic factors. Further trials
are needed to evaluate the role and timing of WBRT
alongside systemic treatment and local therapies. A
prospective study is essential to develop an accurate
prognostic index to guide WBRT versus supportive care
decisions.
REFERENCES
1. Balasubramanian SK, Sharma M, Venur VA, Schmitt P, Kotecha R, Chao ST, et al. Impact of EGFR mutation and ALK rearrangement
on the outcomes of non–small cell lung cancer patients with brain
metastasis. Neuro Oncol. 2020;22:267-77. Crossref
2. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and
pathophysiology. J Neurooncol. 2005;75:5-14. Crossref
3. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast,
colon, kidney, and lung and melanoma. Cancer. 2002;94:2698-705. Crossref
4. Schuette W. Treatment of brain metastases from lung cancer:
chemotherapy. Lung Cancer. 2004;45 Suppl 2:S253-7. Crossref
5. Naresh G, Malik PS, Khurana S, Pushpam D, Sharma V, Yadav M,
et al. Assessment of brain metastasis at diagnosis in non–small-cell
lung cancer: a prospective observational study from North India.
JCO Glob Oncol. 2021;7:593-601. Crossref
6. Soffietti R, Rudà R, Trevisan E. Brain metastases: current
management and new developments. Curr Opin Oncol.
2008;20:676-84. Crossref
7. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE,
Schell MC, et al. Whole brain radiation therapy with or without
stereotactic radiosurgery boost for patients with one to three brain
metastases: phase III results of the RTOG 9508 randomised trial.
Lancet. 2004;363:1665-72. Crossref
8. Mandell L, Hilaris B, Sullivan M, Sundaresan N, Nori D, Kim JH, et al. The treatment of single brain metastasis from non-oat cell lung
carcinoma. Surgery and radiation versus radiation therapy alone.
Cancer. 1986;58:641-9. Crossref
9. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7:682-9. Crossref
10. Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, et al. The palliation of brain metastases: final results of the first two
studies by the Radiation Therapy Oncology Group. Int J Radiat
Oncol Biol Phys. 1980;6:1-9. Crossref
11. Sneed PK, Larson DA, Wara WM. Radiotherapy for cerebral
metastases. Neurosurg Clin N Am. 1996;7:505-15. Crossref
12. Order SE, Hellmän S, Von Essen CF, Kligerman MM. Improvement
in quality of survival following whole-brain irradiation for brain
metastasis. Radiology. 1968;91:149-53. Crossref
13. Brown PD, Stephanie Pugh, Laack NN, Wefel JS, Khuntia D,
Meyers C, et al. Memantine for the prevention of cognitive
dysfunction in patients receiving whole-brain radiotherapy: a
randomized, double-blind, placebo-controlled trial. Neuro Oncol.
2013;15:1429-37. Crossref
14. Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS,
et al. Hippocampal avoidance during whole-brain radiotherapy plus
memantine for patients with brain metastases: phase III Trial NRG
Oncology CC001. J Clin Oncol. 2020;38:1019-29. Crossref
15. Steindl A, Brunner TJ, Heimbach K, Schweighart K, Moser GM,
Niziolek HM, et al. Changing characteristics, treatment approaches
and survival of patients with brain metastasis: data from six
thousand and thirty-one individuals over an observation period of
30 years. Eur J Cancer. 2022;162:170-81. Crossref
16. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P,
McColl E, et al. Dexamethasone and supportive care with or
without whole brain radiotherapy in treating patients with non–small
cell lung cancer with brain metastases unsuitable for resection or
stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority,
randomised trial. Lancet. 2016;388:2004-14. Crossref
17. Sperduto PW, De B, Li J, Carpenter D, Kirkpatrick J, Milligan M,
et al. Graded prognostic assessment (GPA) for patients with lung
cancer and brain metastases: Initial report of the small cell lung
cancer GPA and update of the non–small cell lung cancer GPA
including the effect of programmed death ligand 1 and other
prognostic factors. Int J Radiat Oncol Biol Phys. 2022;114:60-74. Crossref
18. Yao Y, Zhao M, Yuan D, Gu X, Liu H, Song Y. Elevated
pretreatment serum globulin albumin ratio predicts poor prognosis
for advanced non–small cell lung cancer patients. J Thorac Dis.
2014;6:1261-70. Crossref
19. Lu P, Ma Y, Wei S, Liang X. A low albumin-to-globulin ratio
predicts a poor prognosis in patients with metastatic non–small-cell
lung cancer. Front Med (Lausanne). 2021;8:621592. Crossref
20. Huang H, Li L, Luo W, Yang Y, Ni Y, Song T, et al. Lymphocyte percentage as a valuable predictor of prognosis in lung cancer. J Cell Mol Med. 2022;26:1918-31. Crossref
21. Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for
predicting lung cancer prognosis: a meta-analysis of 7219 patients.
Mol Clin Oncol. 2017;7:498-506. Crossref
22. Hong Kong Cancer Registry, Hospital Authority. Lung Cancer in
2021. Oct 2023. Available from: https://www3.ha.org.hk/cancereg/pdf/factsheet/2021/lung_2021.pdf. Accessed 30 May 2024 .
23. Lee HL, Chung TS, Ting LL, Tsai JT, Chen SW, Chiou JF, et al.
EGFR mutations are associated with favorable intracranial response
and progression-free survival following brain irradiation in non–small
cell lung cancer patients with brain metastases. Radiat Oncol.
2012;7:181. Crossref