Breast Lesions in Paediatric and Young Adults: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2025;28:Epub 16 June 2025
Breast Lesions in Paediatric and Young Adults: A Pictorial Essay
EH Chan, SC Woo, CM Chau, WY Fung, TKB Lai, RLS Chan, Y Leng, C Tang, NY Pan, T Wong
Department of Diagnostic and Interventional Radiology, Kowloon West Cluster, Hong Kong SAR, China
Correspondence: Dr EH Chan, Department of Diagnostic and Interventional Radiology, Kowloon West Cluster, Hong Kong SAR,
China. Email: ceh278@ha.org.hk
Submitted: 10 August 2024; Accepted: 25 November 2024. This version may differ from the print version.
Contributors: All authors designed the study. EHC, SCW and TW acquired and analysed the data. EHC drafted the manuscript. SCW, CMC,
WKF, TKBL, RLSC, YL, CT, NYP and TW critically revised the manuscript for important intellectual content. All authors had full access to the
data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, TW was not involved in the peer review process. Other authors have disclosed no conflicts of
interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: PAED-2024-029). The requirement for patient consent was waived by the Board due to the retrospective nature of the study.
INTRODUCTION
Breast lesions can present as palpable lumps in children
and young adults, causing anxiety to patients and their
caregivers. Although malignant lesions are exceedingly
rare in this age-group, familiarity with the spectrum of
breast lesions and the diagnostic approach is crucial
to guide appropriate management. Evaluation and
intervention should be tailored to minimise damage
to developing breast tissue. All patients with breast
abnormalities should first undergo clinical assessment.[1]
When imaging is indicated, ultrasound (US) is
recommended as the initial radiological examination
for females under 30 years of age with palpable breast
masses, according to the American College of Radiology
(ACR) Appropriateness Criteria.[2] Mammography is
less favoured due to the ionising radiation and reduced
sensitivity in dense breast tissues of young patients.
Most benign lesions in young women are not visible
on mammography.[2] [3] Digital breast tomosynthesis
potentially increases lesion detection in overlapping
tissue in young dense breasts. Magnetic resonance
imaging (MRI) is used for defining disease extent, surgical planning, and screening in high-risk females
with hereditary predispositions and prior irradiation.[1] [4]
This pictorial essay showcases both benign and
malignant breast lesions in individuals under 30 years
of age on multimodality imaging, with emphasis on
various MRI presentations as its use in both diagnostic
and screening indications has been rapidly expanding.
Guidelines on screening and risk factors for early-onset
breast cancer, including hereditary predispositions and
prior radiotherapy, are included. The role of radiologists
in follow-up imaging and the appropriate timing for
image-guided intervention, while staying aware of
the risks of iatrogenic injury to developing breasts, is
discussed.
NORMAL BREAST DEVELOPMENT AND VARIANTS
Neonatal Breast Development
Breast development occurs at prenatal and pubertal
stages. At the fourth week of gestation, paired ectodermal
thickenings develop on the ventral surface of the embryo and extend in a line between the axilla and inguinal
regions, forming the mammary crest. This is followed
by involution of the mammary crest at the tenth week of
gestation except at the fourth intercostal spaces, giving
rise to breast buds.[3] [5]
Accessory breast tissue, also known as polymastia,
develops when there is incomplete regression. This can
be found in up to 6% of the population, usually occurring
along the mammary crest and most commonly in the
axilla.[1] Imaging shows heterogeneous fibroglandular
tissue with characteristics similar to normal breast parenchyma[3] [6] (Figure 1). It is crucial to recognise
this variant as it could be affected by the pathological
processes that occur in normal breast tissues.
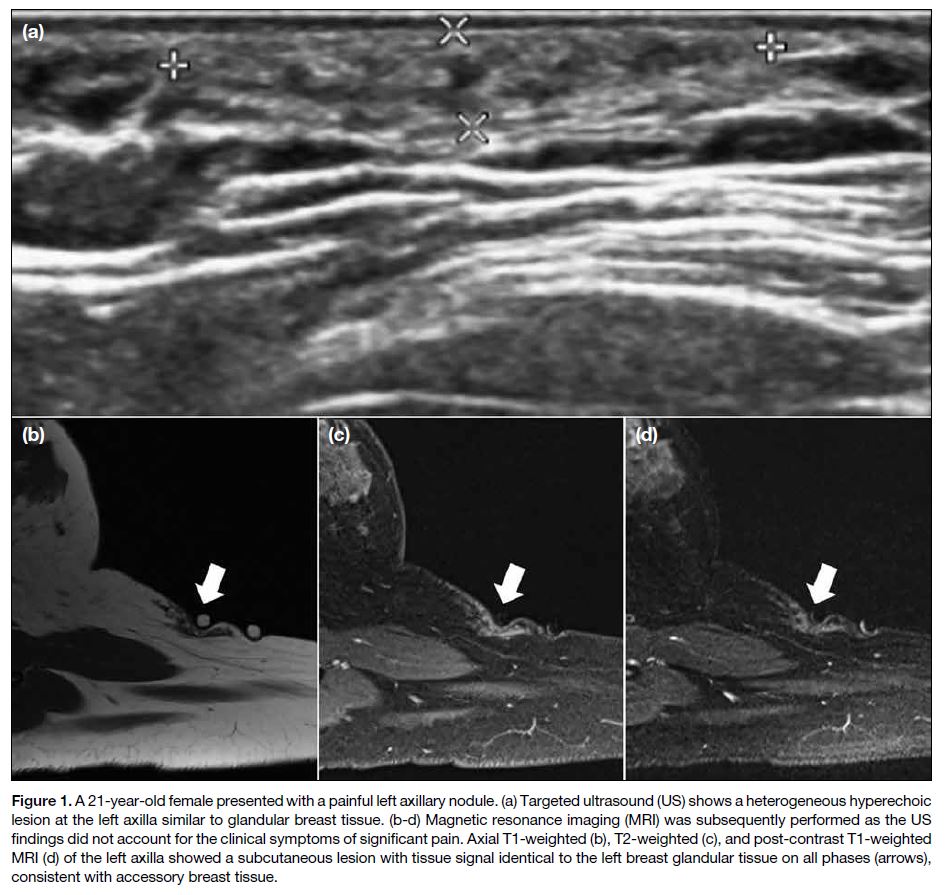
Figure 1. A 21-year-old female presented with a painful left axillary nodule. (a) Targeted ultrasound (US) shows a heterogeneous hyperechoic
lesion at the left axilla similar to glandular breast tissue. (b-d) Magnetic resonance imaging (MRI) was subsequently performed as the US
findings did not account for the clinical symptoms of significant pain. Axial T1-weighted (b), T2-weighted (c), and post-contrast T1-weighted
MRI (d) of the left axilla showed a subcutaneous lesion with tissue signal identical to the left breast glandular tissue on all phases (arrows),
consistent with accessory breast tissue.
Physiological Neonatal Breast Development
Up to 70% of newborns experience physiological breast
development under maternal oestrogen influence.[3] It can
be unilateral or more commonly bilateral. This condition
is transient and usually resolves spontaneously by 6
months of age. Normal breast buds may fluctuate in size
and remain palpable up to 2 years of age, after which
they remain quiescent until puberty.[3] US features include retroareolar hypoechoic tissue (Figure 2), or hyperechoic
nodule with hypoechoic linear structures representing
simple branch ducts.
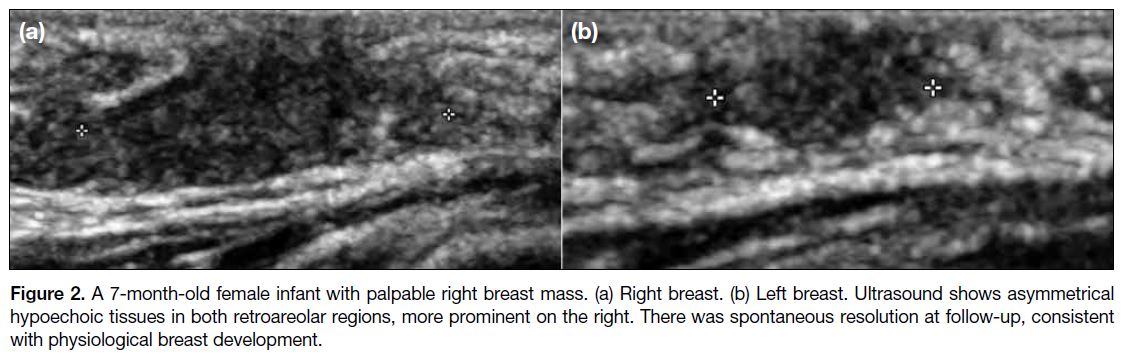
Figure 2. A 7-month-old female infant with palpable right breast mass. (a) Right breast. (b) Left breast. Ultrasound shows asymmetrical
hypoechoic tissues in both retroareolar regions, more prominent on the right. There was spontaneous resolution at follow-up, consistent
with physiological breast development.
Thelarche
During puberty, female breasts develop under the
influence of the secretion of oestrogen and other
hormones. This is known as thelarche, which is divided
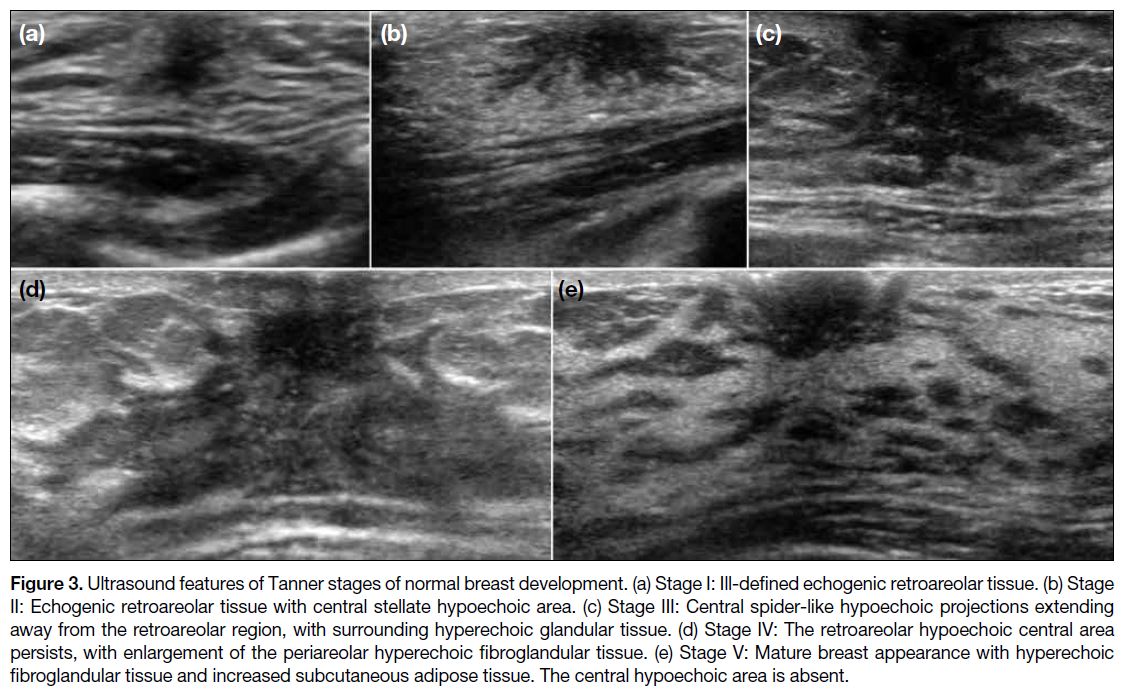
into five stages on the Tanner scale[5] [7] (Figure 3). On
US, stage I shows ill-defined echogenic retroareolar
tissue. In stage II, a central stellate hypoechoic area appears. Stage III shows central spider-like hypoechoic
projections extending out from the retroareolar region,
with surrounding hyperechoic glandular tissue. Stage IV
involves growth of periareolar hyperechoic fibroglandular
tissue with a hypoechoic central area. Finally, stage V
reveals hyperechoic fibroglandular tissue and increased
subcutaneous adipose tissue with disappearance of the
central hypoechoic area.
Figure 3. Ultrasound features of Tanner stages of normal breast development. (a) Stage I: Ill-defined echogenic retroareolar tissue. (b) Stage
II: Echogenic retroareolar tissue with central stellate hypoechoic area. (c) Stage III: Central spider-like hypoechoic projections extending
away from the retroareolar region, with surrounding hyperechoic glandular tissue. (d) Stage IV: The retroareolar hypoechoic central area
persists, with enlargement of the periareolar hyperechoic fibroglandular tissue. (e) Stage V: Mature breast appearance with hyperechoic
fibroglandular tissue and increased subcutaneous adipose tissue. The central hypoechoic area is absent.
Premature Thelarche
Premature thelarche refers to isolated early breast development in girls under 8 years without associated
skeletal maturation.[5] It can be unilateral or bilateral,
symmetrical or asymmetrical. Imaging features are
identical to thelarche, seen as developing breast tissue
without discrete lesion on US.[8]
Gynaecomastia
Gynaecomastia refers to enlargement of male breast
tissue, occurring most frequently during adolescence due
to physiological transient increase in oestrogen levels. It
typically involutes spontaneously when androgen levels
rise.[3] Secondary causes include Klinefelter syndrome;
drug use (e.g., anabolic steroids, exogenous oestrogens,
marijuana); and tumours such as prolactinomas.[3] [5] On
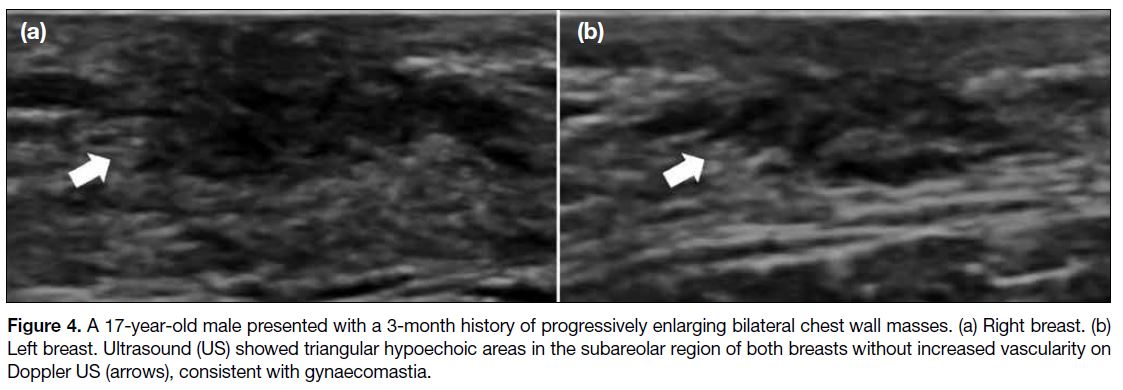
mammography, a flame-shaped retroareolar density is
characteristic, while it is triangular and hypoechoic on
US (Figure 4).[3]
Figure 4. A 17-year-old male presented with a 3-month history of progressively enlarging bilateral chest wall masses. (a) Right breast. (b)
Left breast. Ultrasound (US) showed triangular hypoechoic areas in the subareolar region of both breasts without increased vascularity on
Doppler US (arrows), consistent with gynaecomastia.
NON-NEOPLASTIC LESIONS
Trauma or Surgery-Related
Haematomas should be considered in patients who
present with a new-onset breast lesion after recent
trauma or surgery. They can be solid, cystic, or of mixed
echogenicity on US, and are commonly avascular[1] [3]
(Figure 5). It is crucial to look for the presence of foreign
bodies, as removal may be needed.[3]
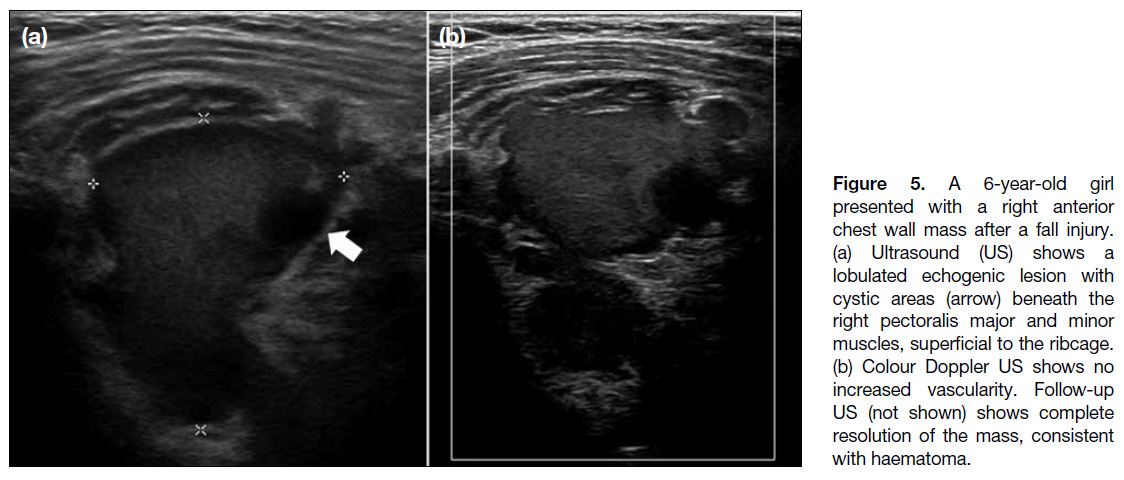
Figure 5. A 6-year-old girl
presented with a right anterior
chest wall mass after a fall injury.
(a) Ultrasound (US) shows a
lobulated echogenic lesion with
cystic areas (arrow) beneath the
right pectoralis major and minor
muscles, superficial to the ribcage.
(b) Colour Doppler US shows no
increased vascularity. Follow-up
US (not shown) shows complete
resolution of the mass, consistent
with haematoma.
Prior breast trauma can also result in fat necrosis, which
may appear as solid masses to oil cysts, depending
on lesion age.[3] [5] On US, they can be hyperechoic,
hypoechoic with posterior acoustic enhancement,
anechoic, or of mixed echogenicity with internal
cystic spaces. With a typical trauma history, follow-up
US in 3 to 6 months is suggested to confirm
resolution.[3]
Galactoceles
Galactoceles are milk retention cysts resulting from
lactiferous duct obstruction. They are predominantly
seen in pregnant or lactating women and are rare in
infants and adolescents. US shows a complex cystic mass
with variable internal echogenicity depending on its fat
and water content. Fat-fluid levels within the lesion are
considered diagnostic (Figure 6).[3] [5]
Figure 6. A 23-year-old lactating female presented with a palpable lump in the left breast at 10 o’clock position. (a) Targeted ultrasound (US)
in transverse plane shows a hypoechoic and anechoic lesion with a fat-fluid level (arrow) and posterior acoustic enhancement. (b) Doppler
US study shows no internal vascularity. Fine needle aspiration confirmed the diagnosis of galactocele.
Cysts
Cysts are uncommon in paediatric patients and are
usually solitary.[1] A cyst appears as an avascular anechoic
lesion with thin wall and posterior acoustic enhancement
on US, indicating benignity. Infected cysts may contain
internal echoes, fluid-fluid levels, thickened walls, and
peripheral hypervascularity.[7]
Infection and Inflammation
Mastitis refers to infection or inflammation of breast
tissue. In the first 2 months of life, mastitis neonatorum
can occur due to mammary ductal obstruction or skin
breaks permitting bacteria seeding.[7] Puerperal mastitis
can affect pregnant or breast-feeding women. The most
common pathogen is Staphylococcus aureus.[7] [8] On
US, mastitis may appear as focal or diffuse ill-defined
heterogeneous hypoechoic and hyperechoic areas, with
overlying skin thickening. Colour Doppler may show
hyperaemia with central flow (Figure 7).[1] [3] [4]
Granulomatous mastitis may be idiopathic or due to
systemic conditions, including autoimmune diseases,
diabetes, or tuberculosis. These should be excluded
before diagnosing idiopathic granulomatous mastitis.
Over 50% of cases showed an irregular hypoechoic
parallel mass with tubular extensions on US (Figure 8).[9]
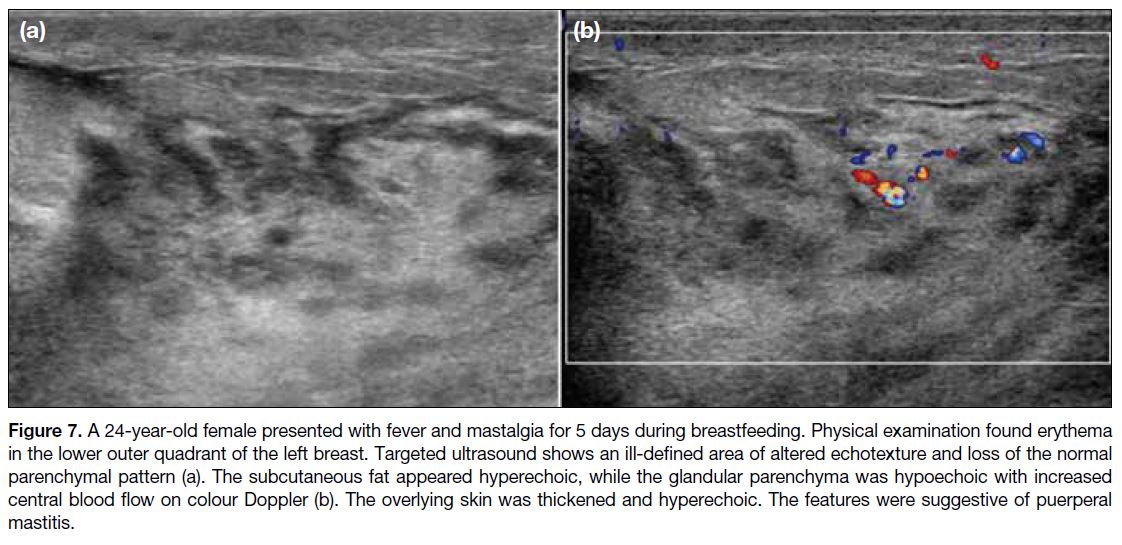
Figure 7. A 24-year-old female presented with fever and mastalgia for 5 days during breastfeeding. Physical examination found erythema
in the lower outer quadrant of the left breast. Targeted ultrasound shows an ill-defined area of altered echotexture and loss of the normal
parenchymal pattern (a). The subcutaneous fat appeared hyperechoic, while the glandular parenchyma was hypoechoic with increased
central blood flow on colour Doppler (b). The overlying skin was thickened and hyperechoic. The features were suggestive of puerperal
mastitis.
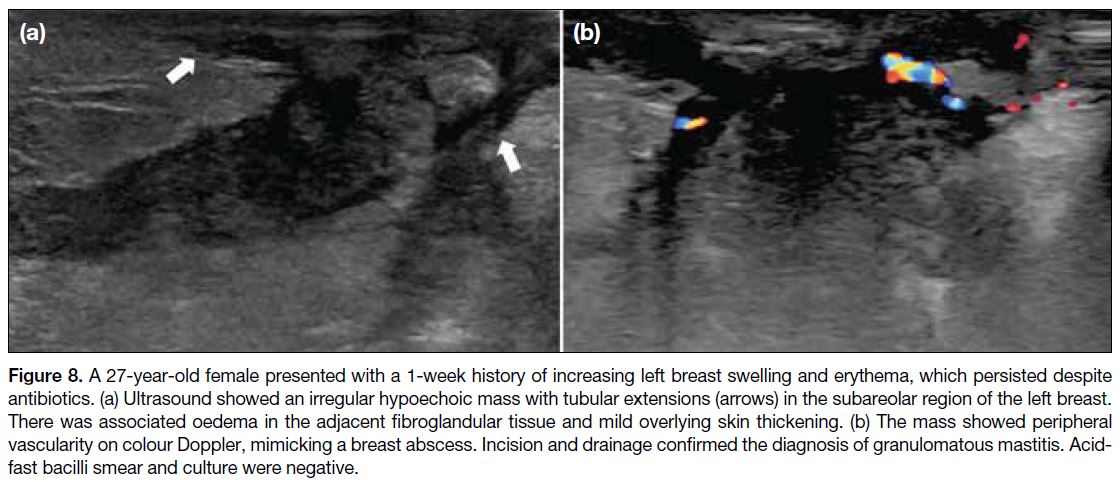
Figure 8. A 27-year-old female presented with a 1-week history of increasing left breast swelling and erythema, which persisted despite
antibiotics. (a) Ultrasound showed an irregular hypoechoic mass with tubular extensions (arrows) in the subareolar region of the left breast.
There was associated oedema in the adjacent fibroglandular tissue and mild overlying skin thickening. (b) The mass showed peripheral
vascularity on colour Doppler, mimicking a breast abscess. Incision and drainage confirmed the diagnosis of granulomatous mastitis. Acid-fast
bacilli smear and culture were negative.
Breast abscesses are often seen as anechoic or hypoechoic
lesions with debris and posterior acoustic enhancement
on US. In contrast to mastitis, abscesses show only
peripheral flow.[7] [8]
Infantile Mammary Duct Ectasia
Infantile mammary duct ectasia refers to retroareolar
ductal dilatation in infants and young children. The exact
cause is unknown.[3] Patients may be asymptomatic or
present with bloody nipple discharge.[3] US demonstrates
a cluster of tubular anechoic structures with or without
internal debris.[3] Associated simple or multiloculated
cystic lesions may also be seen.[3] The condition typically
resolves after breastfeeding ceases.[3]
Intramammary Lymph Nodes
Intramammary lymph nodes, found in the breast and
axillary tail, may become reactive due to inflammation,
infection, or recent vaccination. Suspicious features
include eccentric cortical thickening of more than 3 mm, extracapsular extension, loss of fatty hilum, or non-hilar
blood flow; all require tissue sampling.[3]
NEOPLASTIC LESIONS
Vascular and Lymphatic Tumours
Infantile haemangioma (IH) is the most common
benign neoplasm in infants and can occur in the
breast.[8] [10] It is typically absent at birth, rapidly
proliferates in the first few weeks to months of life and
usually reaches its maximal size by 3 months of age,
then spontaneously involutes from 12 months of age,
with complete regression by 4 years old in most cases.[3] US or MRI are mainly for treatment planning. During
proliferation, IH appears as a well-defined solid mass
with a lobulated border of mixed echogenicity on US,
with marked diffuse increased vascularity (Figure 9),
followed by the plateau phase where it stops enlarging.
Finally, IH decreases in size and vascularity during the
involution phase. Echogenic areas may be identified,
suggestive of fibrofatty tissue. Other vascular tumours
such as congenital haemangioma and tufted angioma
are uncommon.[10]
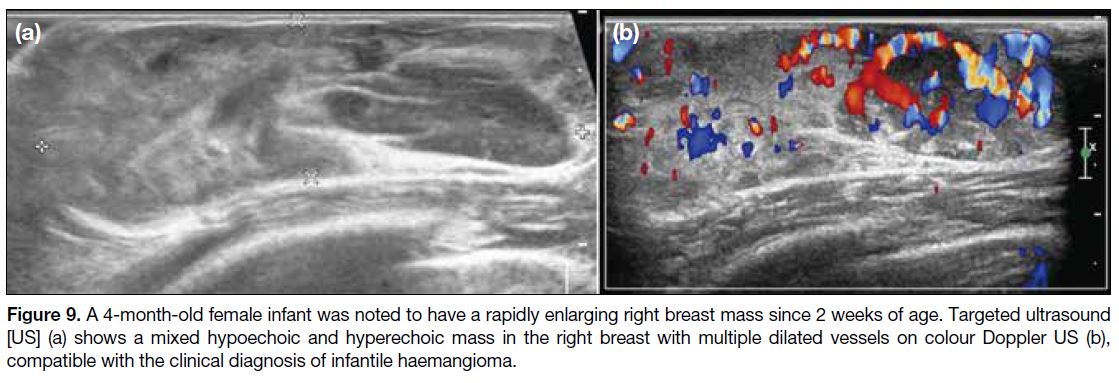
Figure 9. A 4-month-old female infant was noted to have a rapidly enlarging right breast mass since 2 weeks of age. Targeted ultrasound
[US] (a) shows a mixed hypoechoic and hyperechoic mass in the right breast with multiple dilated vessels on colour Doppler US (b),
compatible with the clinical diagnosis of infantile haemangioma.
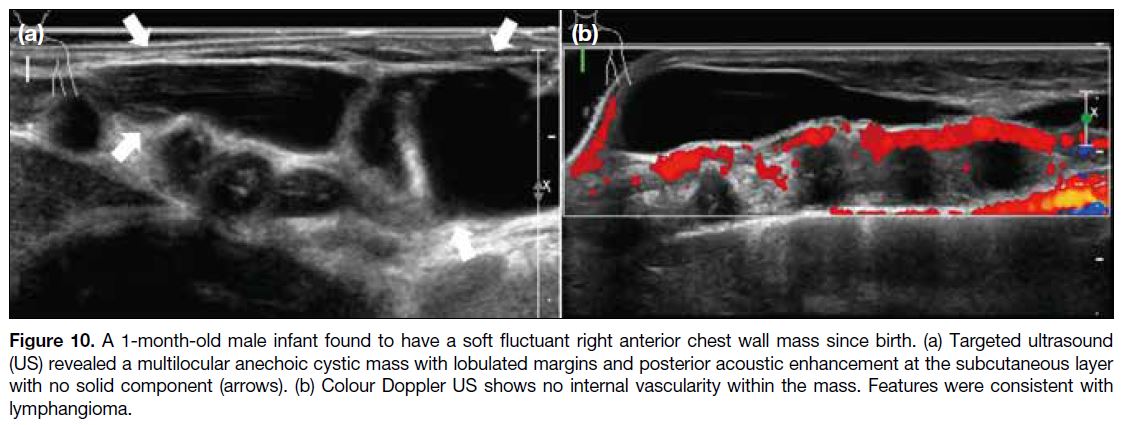
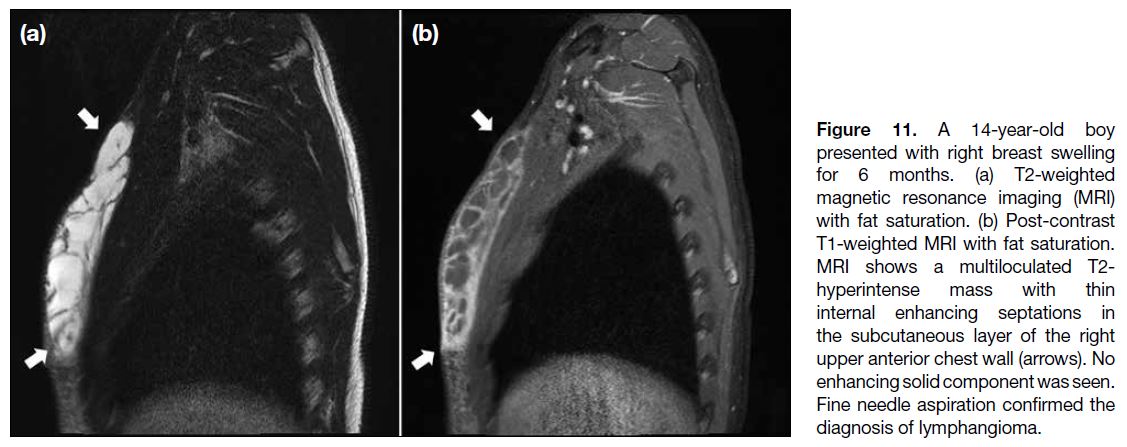
Lymphangiomas are benign developmental lymphatic tumours, most frequently in the neck or axilla, but may
also affect the breast. Usually presenting before 2 years of
age, they appear as avascular compressible multiseptated
cystic masses on US (Figure 10) and T2-hyperintense
lesions without an enhancing solid component on MRI3
(Figure 11).
Figure 10. A 1-month-old male infant found to have a soft fluctuant right anterior chest wall mass since birth. (a) Targeted ultrasound
(US) revealed a multilocular anechoic cystic mass with lobulated margins and posterior acoustic enhancement at the subcutaneous layer
with no solid component (arrows). (b) Colour Doppler US shows no internal vascularity within the mass. Features were consistent with
lymphangioma.
Figure 11. A 14-year-old boy presented with right breast swelling for 6 months. (a) T2-weighted magnetic resonance imaging (MRI) with fat saturation. (b) Post-contrast T1-weighted MRI with fat saturation. MRI shows a multiloculated T2-hyperintense mass with thin internal enhancing septations in the subcutaneous layer of the right upper anterior chest wall (arrows). No enhancing solid component was seen. Fine needle aspiration confirmed the diagnosis of lymphangioma.
Fibroepithelial Lesions
Fibroadenoma is the most common benign fibroepithelial
tumour in females under 30 years of age, arising from
stromal and epithelial tissues and accounting for 54%
to 94% of breast masses in children and adolescents.[5]
Masses reaching 5 cm are termed giant fibroadenomas.[3] [5] Juvenile fibroadenoma is an uncommon variant with
hypercellular stromal proliferation that can grow rapidly
and cause skin distortion.[1] [4] [5] On US, fibroadenoma
typically appears as a well-circumscribed hypoechoic
parallel mass with variable posterior enhancement and
sometimes a pseudocapsule. Fibroadenomas in up to
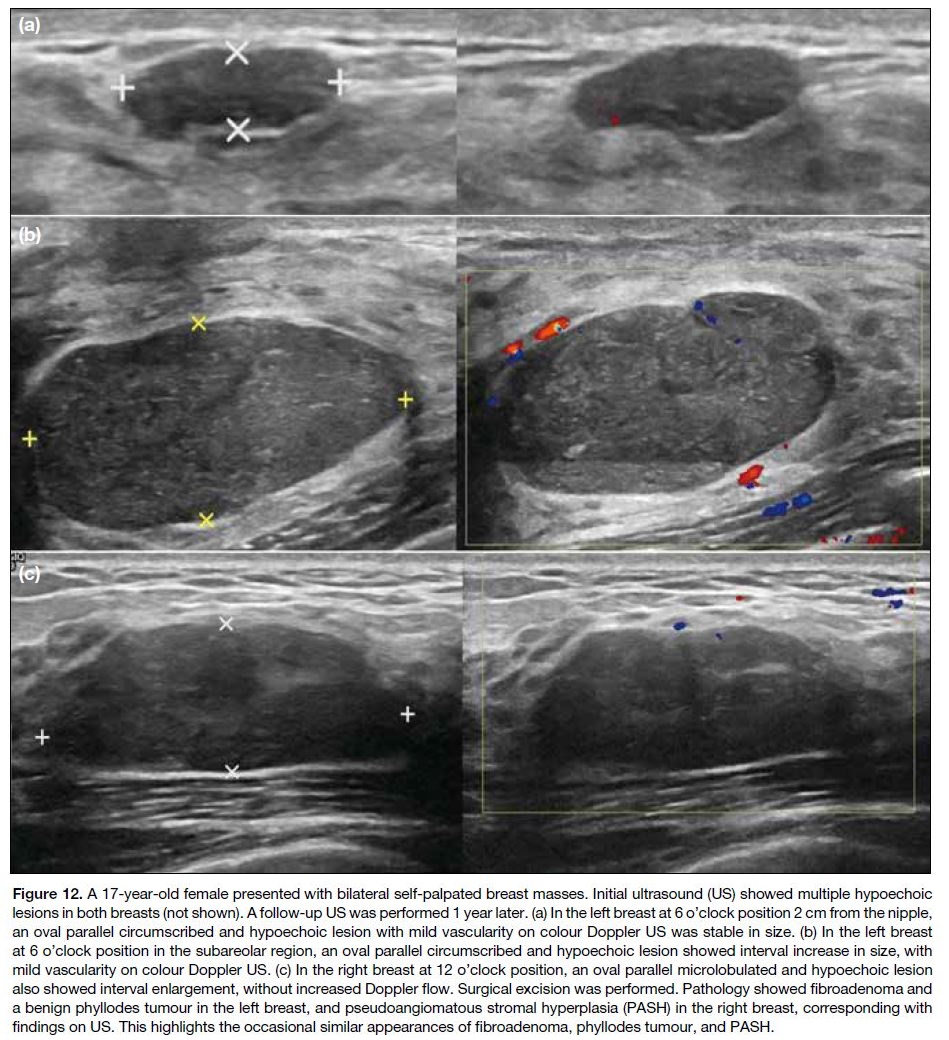
one-third of young breasts are vascular7 (Figure 12a).
Figure 12. A 17-year-old female presented with bilateral self-palpated breast masses. Initial ultrasound (US) showed multiple hypoechoic
lesions in both breasts (not shown). A follow-up US was performed 1 year later. (a) In the left breast at 6 o’clock position 2 cm from the nipple,
an oval parallel circumscribed and hypoechoic lesion with mild vascularity on colour Doppler US was stable in size. (b) In the left breast
at 6 o’clock position in the subareolar region, an oval parallel circumscribed and hypoechoic lesion showed interval increase in size, with
mild vascularity on colour Doppler US. (c) In the right breast at 12 o’clock position, an oval parallel microlobulated and hypoechoic lesion
also showed interval enlargement, without increased Doppler flow. Surgical excision was performed. Pathology showed fibroadenoma and
a benign phyllodes tumour in the left breast, and pseudoangiomatous stromal hyperplasia (PASH) in the right breast, corresponding with
findings on US. This highlights the occasional similar appearances of fibroadenoma, phyllodes tumour, and PASH.
Phyllodes tumour is another fibroepithelial tumour of
cellular stroma with branching leaf-like epithelium-lined
cystic spaces, typically presenting as a rapid
growing mass.[3] It may look sonographically identical
to fibroadenoma, appearing as an oval homogeneous
hypoechoic circumscribed parallel solid mass[3] (Figure 12b). Phyllodes tumours are classified as benign, borderline or malignant subtypes; however, all types
may recur and metastasize, especially to the lungs.[3] In all,
85% of phyllodes tumour in children and adolescents are
benign.[5] As imaging findings and fine needle aspiration
do not distinguish benign from malignant phyllodes
tumour, core needle biopsy is essential.[4] [5] Wide local
excision with negative margins is recommended to
minimise local recurrence.[3]
Pseudoangiomatous Stromal Hyperplasia
Pseudoangiomatous stromal hyperplasia is a rare
benign localised stromal overgrowth, possibly mediated
by hormones.[3] [5] It is usually an incidental finding on
histological analysis but can also present as a lump with
variable sonographic appearance, sometimes seen as an
oval circumscribed hypoechoic or heterogeneous mass
(Figure 12c).[3] [4] [8] Surgery is indicated for symptomatic or
enlarging masses, but recurrence may occur.[3]
Papillomatous Lesions
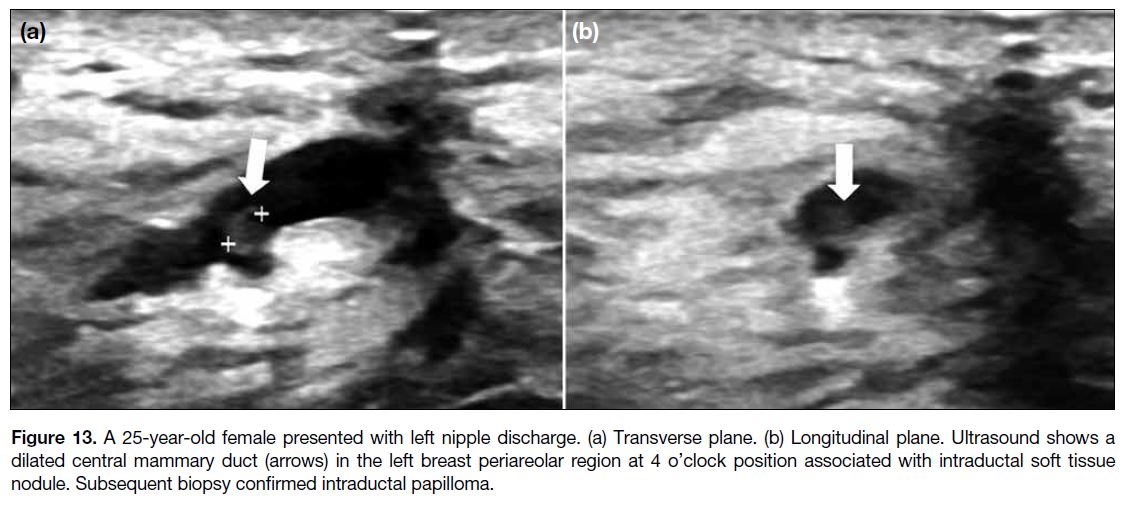
Intraductal papilloma arises from benign epithelial
proliferation of central mammary duct, projecting
into and possibly obstructing the duct, causing nipple
discharge at presentation.[1] It is uncommon in children
and adolescents, and rare in boys.[5] [8] Typically solitary,
it may appear as a well-defined solid nodule within a
dilated duct on US (Figure 13),[3] often with a vascular
feeding pedicle seen on colour Doppler.[11]
Figure 13. A 25-year-old female presented with left nipple discharge. (a) Transverse plane. (b) Longitudinal plane. Ultrasound shows a
dilated central mammary duct (arrows) in the left breast periareolar region at 4 o’clock position associated with intraductal soft tissue
nodule. Subsequent biopsy confirmed intraductal papilloma.
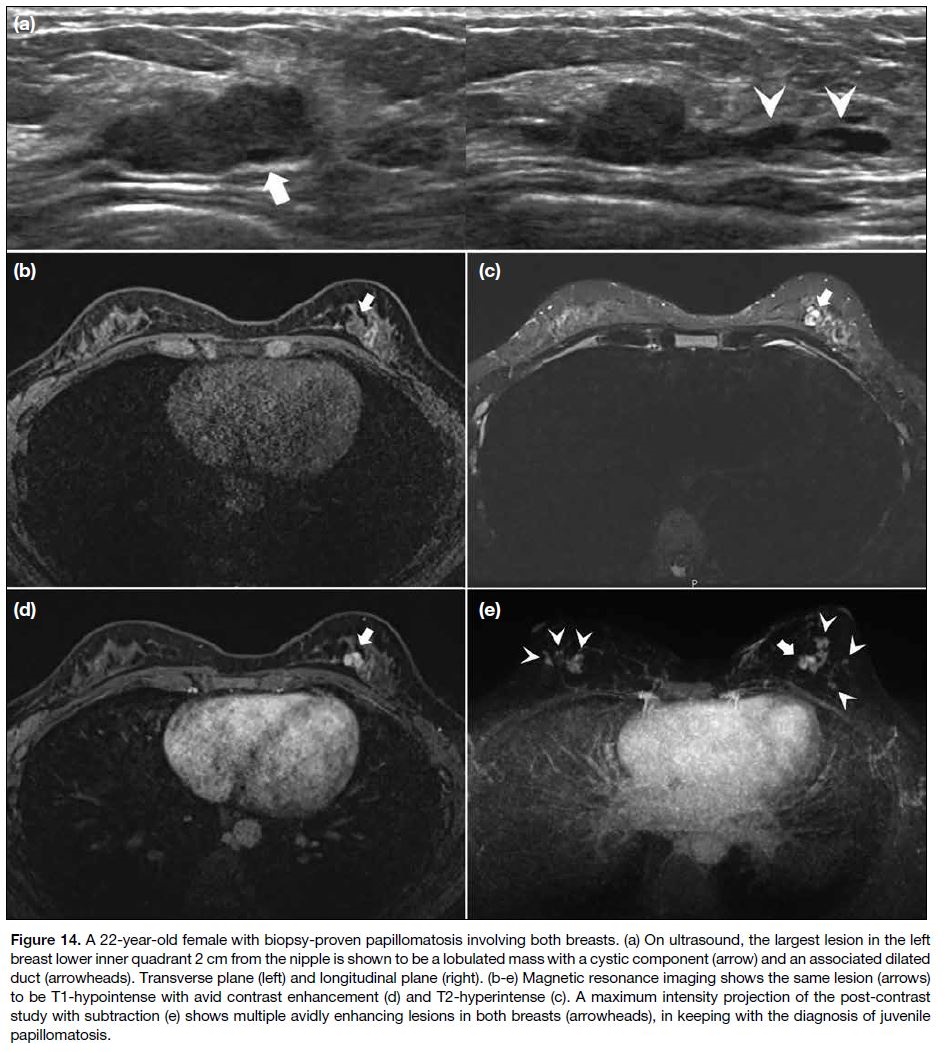
Juvenile papillomatosis occurs when there is localised
proliferation with multiple papillomas in the peripheral
ducts. Unlike intraductal papilloma, there is no fibrovascular core.[3] Ill-defined hypoechoic masses are
seen on US; the presence of multiple peripheral cystic
spaces with a ‘Swiss cheese’ appearance hints at the
diagnosis.[4] On MRI, they are T1 hypointense showing
avid enhancement, with internal T2-hyperintense cystic
spaces (Figure 14).[1] [3] Although juvenile papillomatosis is
benign, up to 80.4% of patients have coexisting atypical
or neoplastic lesions, and it is a marker of familial breast
cancer.[3] [11] This signifies the importance of close follow-up
screening given the increased lifetime breast cancer
risk.[1] [3] [4] [5]
Figure 14. A 22-year-old female with biopsy-proven papillomatosis involving both breasts. (a) On ultrasound, the largest lesion in the left
breast lower inner quadrant 2 cm from the nipple is shown to be a lobulated mass with a cystic component (arrow) and an associated dilated
duct (arrowheads). Transverse plane (left) and longitudinal plane (right). (b-e) Magnetic resonance imaging shows the same lesion (arrows)
to be T1-hypointense with avid contrast enhancement (d) and T2-hyperintense (c). A maximum intensity projection of the post-contrast
study with subtraction (e) shows multiple avidly enhancing lesions in both breasts (arrowheads), in keeping with the diagnosis of juvenile
papillomatosis.
Primary Breast Cancer
Primary breast cancer is rare in paediatrics and young
adults. It accounts for approximately 0.1 case per million
in females younger than 20 years old, and even less in
males.[1] Approximately half of the patients under 30 years
old with breast cancer harbour a germline mutation, such
as BRCA1/2, TP53 for Li-Fraumeni syndrome, and PTEN
for Cowden syndrome.[12] Hence, the diagnosis of breast
cancer in young patients should prompt genetic testing
and counselling.[1] [3] Individuals over the age of 25 years
from a family with known BRCA1/2 mutation carriers
should undergo genetic testing. All females with a
lifetime breast cancer risk of over 20% are recommended
to begin undergoing annual screening MRIs from the age
of 25 years with additional annual mammography from
the age of 30 years.[13]
In 2020, the International Guideline Harmonization
Group recommends breast cancer screening in females
with a history of chest radiotherapy with radiation dose of over 10 Gy, or previous upper abdominal
radiotherapy, given the increased risk for breast cancer.[14]
They include childhood cancer survivors such as those
with supradiaphragmatic Hodgkin lymphoma who
underwent chest irradiation, and haematopoietic cell
transplant recipients who had total body irradiation. The elevated risk begins 8 years after treatment and remains
increased beyond 40 years.[14] These cancer survivors who
develop breast cancer after radiotherapy are reported
to have higher mortalities than women with de novo
breast cancer in the general population.[14] The National
Comprehensive Cancer Network Clinical Guidelines suggest that annual breast MRI and mammography
should begin 10 years after treatment, but not before
age 25 years and 30 years, respectively, while the
Children’s Oncology Group Guidelines (2018 version
5) recommend annual mammography and breast MRI to
commence 8 years after treatment or at 25 years of age,
whichever is later.[14]
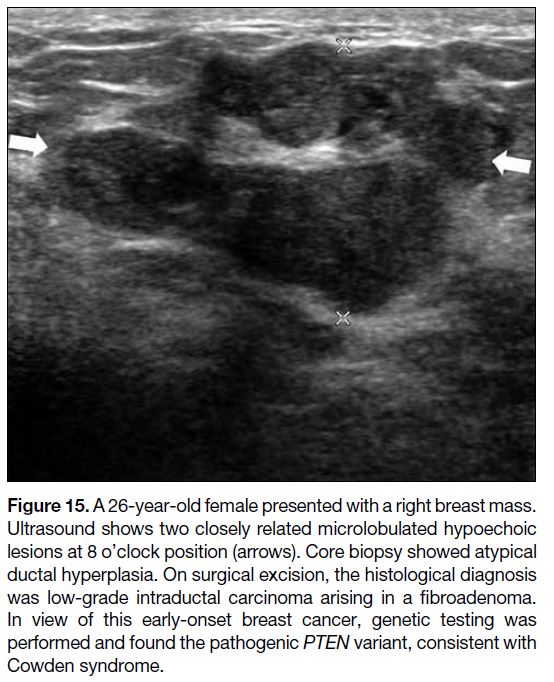
Radiological features considered suspicious in
paediatrics are no different from adults. On US,
concerning features include spiculated margins,
microlobulation, marked hypoechogenicity, and
not being parallel to the chest wall (Figure 15). On
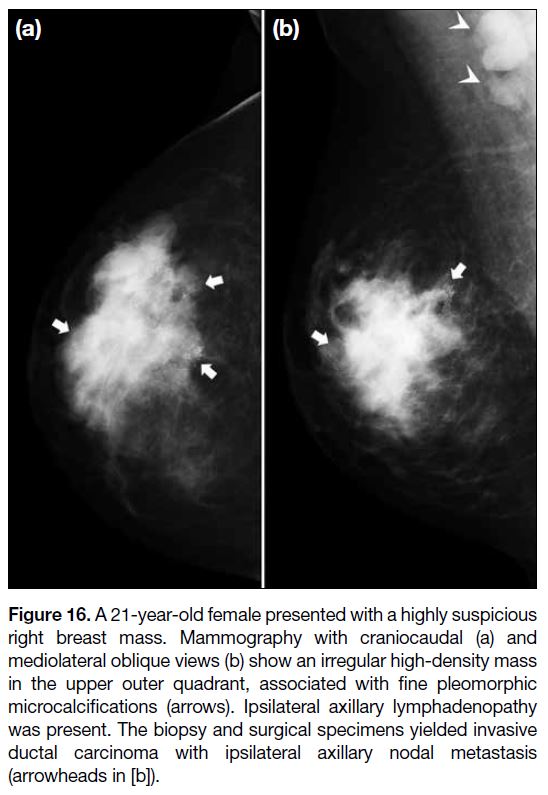
mammography, an irregular, high-density mass with
spiculated or indistinct borders; and microcalcifications
with fine pleomorphic, linear, or linear branching
morphology; and linear or segmental distribution are
worrisome for malignancy (Figure 16). Suspicious
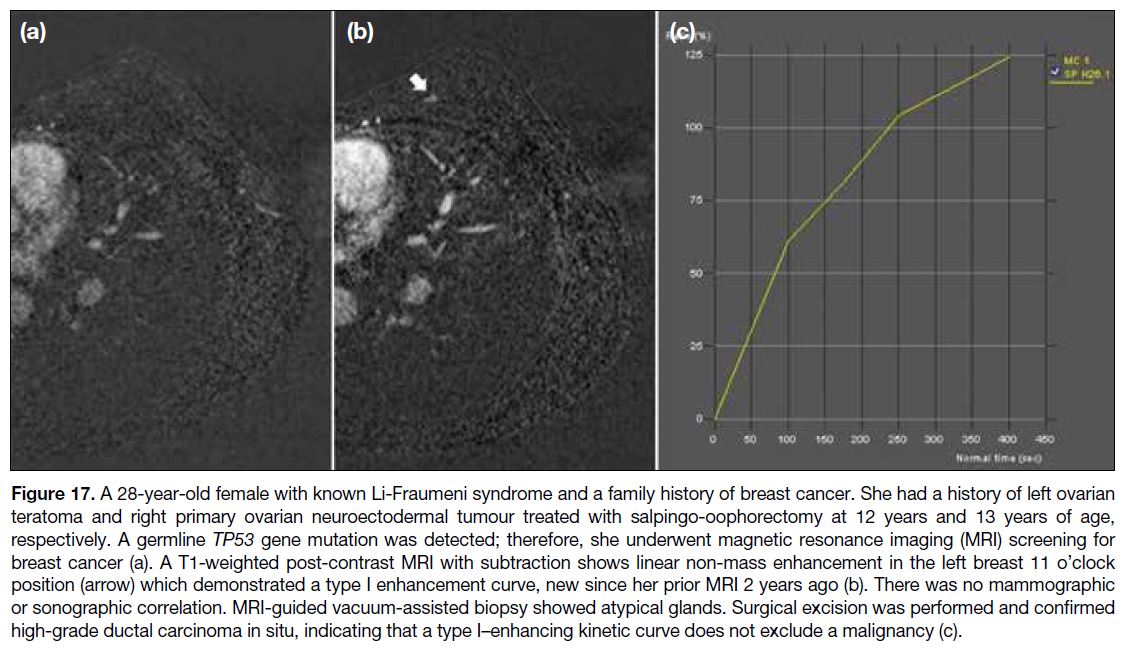
MRI findings include an irregular mass with spiculated
margins and heterogeneous enhancement; or clumped,
heterogeneous, or homogeneous non-mass enhancement
with linear or segmental distribution; and plateau or
washout enhancement kinetics. However, there is
overlap of enhancement kinetics between benign and
malignant lesions, and persistent enhancement cannot
exclude malignancy[15] (Figure 17).
Figure 15. A 26-year-old female presented with a right breast mass.
Ultrasound shows two closely related microlobulated hypoechoic
lesions at 8 o’clock position (arrows). Core biopsy showed atypical
ductal hyperplasia. On surgical excision, the histological diagnosis
was low-grade intraductal carcinoma arising in a fibroadenoma.
In view of this early-onset breast cancer, genetic testing was
performed and found the pathogenic PTEN variant, consistent with
Cowden syndrome.
Figure 16. A 21-year-old female presented with a highly suspicious
right breast mass. Mammography with craniocaudal (a) and
mediolateral oblique views (b) show an irregular high-density mass
in the upper outer quadrant, associated with fine pleomorphic
microcalcifications (arrows). Ipsilateral axillary lymphadenopathy
was present. The biopsy and surgical specimens yielded invasive
ductal carcinoma with ipsilateral axillary nodal metastasis
(arrowheads in [b]).
Figure 17. A 28-year-old female with known Li-Fraumeni syndrome and a family history of breast cancer. She had a history of left ovarian
teratoma and right primary ovarian neuroectodermal tumour treated with salpingo-oophorectomy at 12 years and 13 years of age,
respectively. A germline TP53 gene mutation was detected; therefore, she underwent magnetic resonance imaging (MRI) screening for
breast cancer (a). A T1-weighted post-contrast MRI with subtraction shows linear non-mass enhancement in the left breast 11 o’clock
position (arrow) which demonstrated a type I enhancement curve, new since her prior MRI 2 years ago (b). There was no mammographic
or sonographic correlation. MRI-guided vacuum-assisted biopsy showed atypical glands. Surgical excision was performed and confirmed
high-grade ductal carcinoma in situ, indicating that a type I–enhancing kinetic curve does not exclude a malignancy (c).
Other Breast Malignancies
Breast metastases are more common than primary breast
malignancies in paediatric patients, most frequently
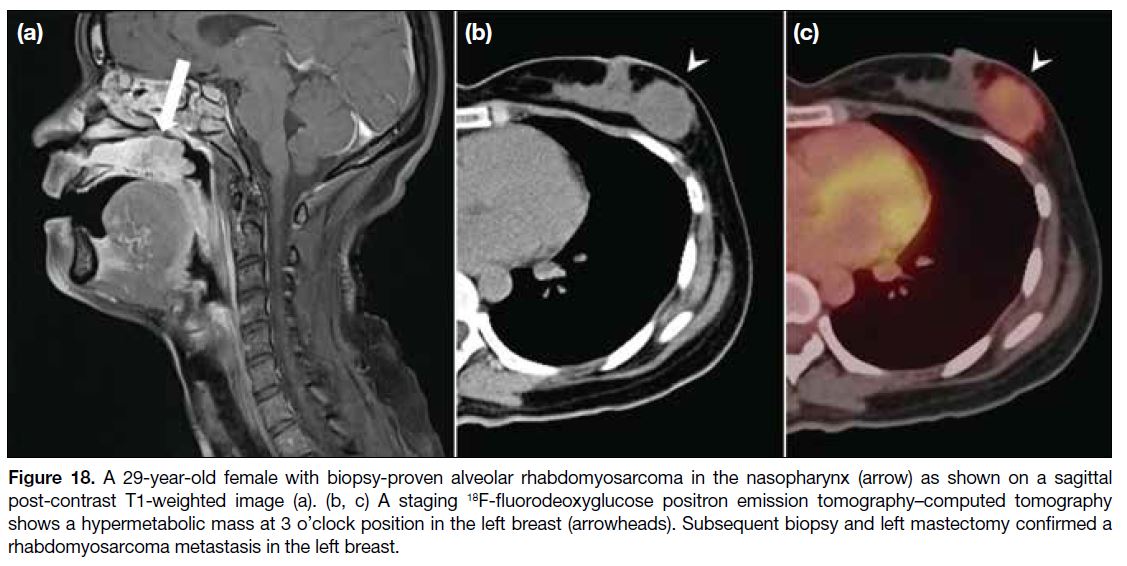
from rhabdomyosarcoma (Figure 18), followed by
neuroblastoma, haematological malignancies including
lymphoma and leukaemia, and Ewing sarcoma.[1] [3] [4]
Breast metastases are usually large and solitary with
variable US features, which can be irregular or lobulated,
heterogeneous, and hypoechoic with hyperechoic
foci.[1] [8] Rhabdomyosarcoma and Ewing sarcoma can
also involve the breast directly as a primary chest wall
malignancy, where evaluation of disease extent with
cross-sectional imaging is often helpful.[1] [3] Lymphoma,
most commonly non–Hodgkin lymphoma, can affect the
breast and ipsilateral axillary lymph nodes primarily, but
is exceedingly rare due to the lack of lymphoid tissue in
the breast.[3]
Figure 18. A 29-year-old female with biopsy-proven alveolar rhabdomyosarcoma in the nasopharynx (arrow) as shown on a sagittal
post-contrast T1-weighted image (a). (b, c) A staging 18F-fluorodeoxyglucose positron emission tomography–computed tomography
shows a hypermetabolic mass at 3 o’clock position in the left breast (arrowheads). Subsequent biopsy and left mastectomy confirmed a
rhabdomyosarcoma metastasis in the left breast.
Next Step of Management: When to Biopsy
According to the ACR Appropriateness Criteria,[2] US is
the most appropriate radiological procedure for initial
evaluation of palpable breast masses in females under 30 years of age. Lesions with benign US features can be
followed up clinically. Sonographic features of benign
breast lesions include circumscribed margins, orientation
parallel to the skin, and less than three gentle smooth
lobulations. Short interval follow-up is recommended
for probably benign lesions.[2]
Developing breast buds in paediatric patients are
vulnerable to injury from biopsy, with potential long-term
consequences including permanent disfiguration.
Therefore, image-guided biopsy should be carefully
considered and discussed. Biopsy should be reserved
for probably benign masses smaller than 4 cm showing atypical US features or rapid enlargement, probably
benign masses that are larger than 4 cm, or masses that
demonstrate malignant features on US.[1] In high-risk
patients with known genetic mutations, prior irradiation,
or extramammary malignancies presenting with an
enlarging breast mass, biopsy should be considered
even if the US findings appear benign.[1] [3] Core biopsy
is preferred over fine needle aspiration due to higher
sensitivity, specificity, and accuracy in histological
grading, while tumour receptor status can also be tested.[2]
Surgical excision may be indicated for rapidly enlarging
or symptomatic breast masses even if they show benign
radiological features or biopsy results, as phyllodes
tumours cannot be excluded.[1]
CONCLUSION
The majority of breast lesions in females under 30
years of age are benign, but malignancies do occur.
Radiologists must be familiar with the diagnostic
approach and able to identify lesions suitable for follow-up
to minimise unnecessary intervention. Prior to
biopsy, the potential long-term consequences on breast
development in young patients must be considered. When
early-onset breast cancer is suspected or diagnosed, it is
important not only to review the patient’s medical history
but also to explore possible hereditary predispositions.
REFERENCES
1. Gao Y, Saksena MA, Brachtel EF, terMeulen DC, Rafferty EA.
How to approach breast lesions in children and adolescents. Eur J
Radiol. 2015;84:1350-64. Crossref
2. Expert Panel on Breast Imaging; Moy L, Heller SL, Bailey L,
D’Orsi C, DiFlorio RM, et al. ACR Appropriateness Criteria ®
Palpable Breast Masses. J Am Coll Radiol. 2017;14(5S):S203-24. Crossref
3. Harper LK, Simmons CL, Woodard GA, Solanki MH, Bhatt AA. Pictorial review of common and uncommon pediatric breast lesions.
Radiographics. 2023;43:e220117. Crossref
4. Kaneda HJ, Mack J, Kasales CJ, Schetter S. Pediatric and adolescent
breast masses: a review of pathophysiology, imaging, diagnosis,
and treatment. AJR Am J Roentgenol. 2013;200:W204-12. Crossref
5. Lee EJ, Chang YW, Oh JH, Hwang J, Hong SS, Kim HJ. Breast
lesions in children and adolescents: diagnosis and management.
Korean J Radiol. 2018;19:978-91. Crossref
6. DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue:
basic information every radiologist should know. AJR Am J
Roentgenol. 2014;202:1157-62. Crossref
7. García CJ, Espinoza A, Dinamarca V, Navarro O, Daneman A,
García H, et al. Breast US in children and adolescents. Radiographics.
2000;20:1605-12. Crossref
8. Chung EM, Cube R, Hall GJ, González C, Stocker JT, Glassman LM.
From the archives of the AFIP: Breast masses in children and
adolescents: radiologic-pathologic correlation. Radiographics.
2009;29:907-31. Crossref
9. Pluguez-Turull CW, Nanyes JE, Quintero CJ, Alizai H, Mais DD,
Kist KA, et al. Idiopathic granulomatous mastitis: manifestations
at multimodality imaging and pitfalls. Radiographics. 2018;38:330-56. Crossref
10. Inarejos Clemente EJ, Diaz Leyva J, Karakas SP, Duarte AM,
Mas TR, Restrepo R. Radiologic and clinical features of infantile
hemangioma: potential pitfalls and differential diagnosis.
Radiographics. 2023;43:e230064. Crossref
11. Eiada R, Chong J, Kulkarni S, Goldberg F, Muradali D. Papillary
lesions of the breast: MRI, ultrasound, and mammographic
appearances. AJR Am J Roentgenol. 2012;198:264-71. Crossref
12. Cathcart-Rake EJ, Ruddy KJ, Bleyer A, Johnson RH. Breast cancer
in adolescent and young adult women under the age of 40 years.
JCO Oncol Pract. 2021;17:305-13. Crossref
13. Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ,
Gilbert F, et al. Prevention and screening in BRCA mutation carriers
and other breast/ovarian hereditary cancer syndromes: ESMO
clinical practice guidelines for cancer prevention and screening.
Ann Oncol. 2016;27(suppl 5):v103-10. Crossref
14. Gao Y, Perez CA, Chhor C, Heller SL. Breast cancer screening in
survivors of childhood cancer. Radiographics. 2023;43:e220155. Crossref
15. Macura KJ, Ouwerkerk R, Jacobs MA, Bluemke DA. Patterns of
enhancement on breast MR images: interpretation and imaging
pitfalls. Radiographics. 2006;26:1719-34. Crossref