Thirteen Years’ Experience of Stereotactic Body Radiation Therapy for Ultra-Central Lung Tumours in Hong Kong
ORIGINAL ARTICLE
Hong Kong J Radiol 2025;28:Epub 18 June 2025
Thirteen Years’ Experience of Stereotactic Body Radiation Therapy for Ultra-Central Lung Tumours in Hong Kong
JKW Ng, MTY Kam, KKS Wong, JQ Du, ECY Wong, RMW Yeung
Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr KW Ng, Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email: njk540@ha.org.hk
Submitted: 14 August 2024; Accepted: 6 December 2024. This version may differ from the final version when published in an issue.
Contributors: JKWN and MTYK designed the study. JKWN acquired and analysed the data, and drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-319-5). The requirement of patient consent was waived by the Board due to the retrospective nature of the research.
Abstract
Introduction
Stereotactic body radiation therapy (SBRT) for ultra-central lung tumours is controversial given the
proximity of the tumours to critical organs at risk (OARs). We undertook a retrospective review of the efficacy and
safety outcomes of ultra-central lung SBRT at a major cancer centre in Hong Kong.
Methods
We analysed patients with either primary or oligometastatic ultra-central lung tumours treated with SBRT
from 2009 to 2022. The primary outcome was local progression-free survival. Secondary outcomes included the
incidence of grade ≥2 SBRT-related toxicity and overall survival (OS). Clinical and dosimetric factors were collected
and analysed for potential associations with survival outcomes.
Results
A total of 66 patients were included. Twenty-four cases were primary lung tumours and 42 were lung
metastases, with the majority of metastatic lesions being of lung origin (n = 32). Indications for SBRT for lung
metastases included oligoprogression (n = 23), oligoresidual disease (n = 13), and oligorecurrence (n = 6). Most
patients (86%) received 50 Gy in five fractions. Median follow-up was 54 months, and median OS was 59 months.
The 1-year and 3-year local failure-free survival rates were 98% and 88%, respectively. Grade 3 and grade 5
toxicity rates were 4.5% and 6%, respectively. A higher dose to 4 cc of the proximal bronchial tree and tumours
located within 1 cm of the mainstem bronchus were associated with grade ≥2 airway toxicity. Oesophageal mean and
maximum doses, and dose to 5 cc of the oesophagus were positively associated with grade ≥2 oesophageal toxicity.
Conclusion
We demonstrated high rates of local control and acceptable toxicity outcomes with ultra-central lung
SBRT. Further results from prospective studies may clarify the optimal dose fractionation and OAR constraints for
this population.
Key Words: Lung neoplasms; Organs at risk; Radiosurgery
中文摘要
香港使用軀體立體定位放射治療超中央型肺腫瘤的十三年經驗
吳珈瑋、甘子揚、黃嘉誠、杜綺鈞、王晉彥、楊美雲
引言
由於超中央型肺腫瘤接近關鍵的危及器官,因此使用軀體立體定位放射治療(SBRT)這腫瘤具爭議性。我們在香港一所主要癌症中心進行回顧性研究,檢視超中央型肺腫瘤的治療效用及安全性。
方法
我們分析了於2009至2022年期間接受SBRT治療的原位或寡轉移超中央型肺腫瘤患者。主要結果為局部無惡化存活期,次要結果為二級或以上、與SBRT相關的毒性發生率及整體存活期。我們收集並分析了臨床及劑量因素,以找出與存活結果有關的潛在關聯。
結果
本研究共包括66名患者,24名患有原位肺腫瘤,42名出現肺轉移,大部分轉移性病變源自肺部(n = 32)。SBRT治療肺轉移的適應症包括寡進展(n = 23)、寡殘留疾病(n = 13)及寡復發(n = 6)。大部分患者(86%)分五次接受劑量為50 Gy的治療。隨訪中位數為54個月,整體存活期中位數為59個月。一年及三年局部無疾病存活率分別為98%及88%。三級及五級毒性比率分別為4.5%及6%。用於受照射的4 cc近端支氣管樹體積的較高劑量以及位於主支氣管1厘米內的腫瘤與二級或以上毒性相關。食道平均及最高劑量以及用於受照射的5 cc食道體積的劑量與二級或以上食道毒性呈正相關。
結論
研究結果顯示超中央型肺部SBRT的局部控制率高,而且毒性結果可接受。未來可研究釐清適用於相關患者的最佳分次劑量及危及器官的限制。
INTRODUCTION
Stereotactic body radiation therapy (SBRT) is an
established treatment for medically inoperable
early-stage non–small-cell lung cancer and has been
increasingly utilised for treatment of oligometastatic
or oligoprogressive lung metastases. In early-stage
peripherally located lung tumours, SBRT confers
high rates of local control, cancer specificity, and
overall survival (OS) with a low incidence of severe
toxicity.[1] However, the safety of SBRT to ‘ultra-central’
lesions, where the gross tumour volume (GTV)
and/or planning target volume (PTV) overlaps critical
mediastinal structures such as the central airway or
oesophagus, remains a matter of debate.[2] This subgroup
was underrepresented in the RTOG 0813 trial where
ultra-central tumours comprised only 17% of the study
population.[2] Alarmingly high rates of fatal airway
bleeding (12%) were also reported in the phase II HILUS
trial.[3]
SBRT for ultra-central lung tumours has been performed
in our institution, a major cancer centre in Hong Kong,
since its introduction in 2009. We sought to undertake a
retrospective review of the efficacy and safety outcomes of ultra-central lung SBRT at our centre.
METHODS
Study Design and Patient Population
All consecutive cases of primary or oligometastatic
ultra-central lung tumours treated with SBRT from 2009
to 2022 at Pamela Youde Nethersole Eastern Hospital
were included for analysis. Patients with incomplete or
missing clinical/dosimetric data were excluded. Ultra-central
tumours were defined as lesions with the PTV
overlapping the trachea, proximal bronchial tree (PBT)
or oesophagus.
Procedures
Patients were simulated with arms above their head
with arm/shoulder supports and immobilised with
the BodyFIX system (Elekta, Stockholm, Sweden).
Expiratory breath hold was used for lobe tumours while
four-dimensional computed tomography simulation
was used for upper/middle lobe tumours or for patients
unable to cooperate with the breath-hold procedure.
Respiratory motion was monitored using a real-time
position management system (Varian; Varian Medical
Systems, Palo Alto [CA], US). An additional intravenous contrast-enhanced simulation scan was performed for
tumours adjacent to mediastinal structures; oral contrast
was used at the discretion of the treating physician.
GTV was delineated in the pulmonary window
(window width = 1600 Hounsfield unit [HU] and
window level = -600 HU), supplemented with images
acquired in the soft tissue window (window width = 400 HU, window level = 20 HU). An internal target
volume was generated from 4D-CT simulation scans and
an isotropic margin of 5 mm expanded from the internal
target volume to form the PTV. For cases undergoing
breath hold, the GTV-to-PTV margin was 8 mm. No
clinical target volume was used.
Dose fractionation schemes included 50 Gy in five
fractions (57 cases), 60 Gy in eight fractions (seven
cases), or 35 Gy in five fractions (two cases). Treatments
were administered on alternating days.
Static-field dynamic intensity modulated radiotherapy
and/or volumetric modulated arc therapy with 6–mega-voltage
photons were used. The prescription isodose
level was chosen such that 95% of the PTV received
the prescribed dose and 99% of the PTV received ≥90%
of the prescribed dose. The prescribed isodose ranged
between 80% and 90% for all plans. Dose constraints
were adapted from the RTOG 0813 protocol[2] and the
American Association of Physicists in Medicine Task
Group 101 report.[4] The maximum point doses (Dmax) to
the trachea, the PBT and the oesophagus were limited to
105% of the prescription dose.
Treatment was delivered with linear accelerators
(TrueBeam; Varian Medical Systems, Palo Alto
[CA], US), with pretreatment cone-beam computed
tomography images obtained before each treatment.
Online verificiation and matching were performed.
Systemic therapies (excluding hormonal treatments)
were withheld at least 24 hours before and after SBRT.
All patients underwent computed tomography scans
of the thorax at 6-month intervals for at least 3 years.
Clinical follow-up and need for additional imaging were
performed at the discretion of treating clinicians.
Outcomes
The primary outcome analysed was local progression-free
survival (PFS). Secondary outcomes included the
incidence of grade ≥2 SBRT-related toxicity—classified
according to the Common Terminology Criteria for Adverse Events version 5.0 grading system[5]—and
OS. Clinical and dosimetric factors were collected
and analysed for potential associations with survival
outcomes.
Statistical Analyses
Local PFS and OS rates were calculated using the
Kaplan–Meier method. Local PFS was defined as the
time from the date of the first SBRT fraction to either
local progression or last follow-up. OS was defined as
the time from SBRT to death from any cause or last
follow-up.
Clinical and dosimetric variables were analysed using
descriptive statistics. Categorical data were represented
as numbers with percentages, while continuous data
were reported as medians with interquartile ranges. All
dosimetric parameters were converted to equivalent
doses of 2-Gy fractions (alpha-beta ratio = 3, for
dosimetric parameters of organs at risk only) using the
linear-quadratic model for comparison across different
dose fractionations.
Comparison of clinical and dosimetric parameters
between patients with or without grade ≥2 toxicity was
done with Chi squared/Fisher’s exact test for categorical
variables, and the Mann-Whitney U test for continuous
variables.
Simple Cox proportional hazards regression analyses
were conducted to identify potential associations
between clinical/dosimetric variables and survival
outcomes. Variables with a p value < 0.1 were entered
into multivariable analysis. A p value of < 0.05 was
considered statistically significant.
Statistical analyses were performed using commercial
software SPSS (Windows version 27.0; IBM Corp,
Armonk [NY], US). The STROBE (Strengthening the
Reporting of Observational Studies in Epidemiology)
checklist was followed in the preparation of the study.
RESULTS
Patient Population
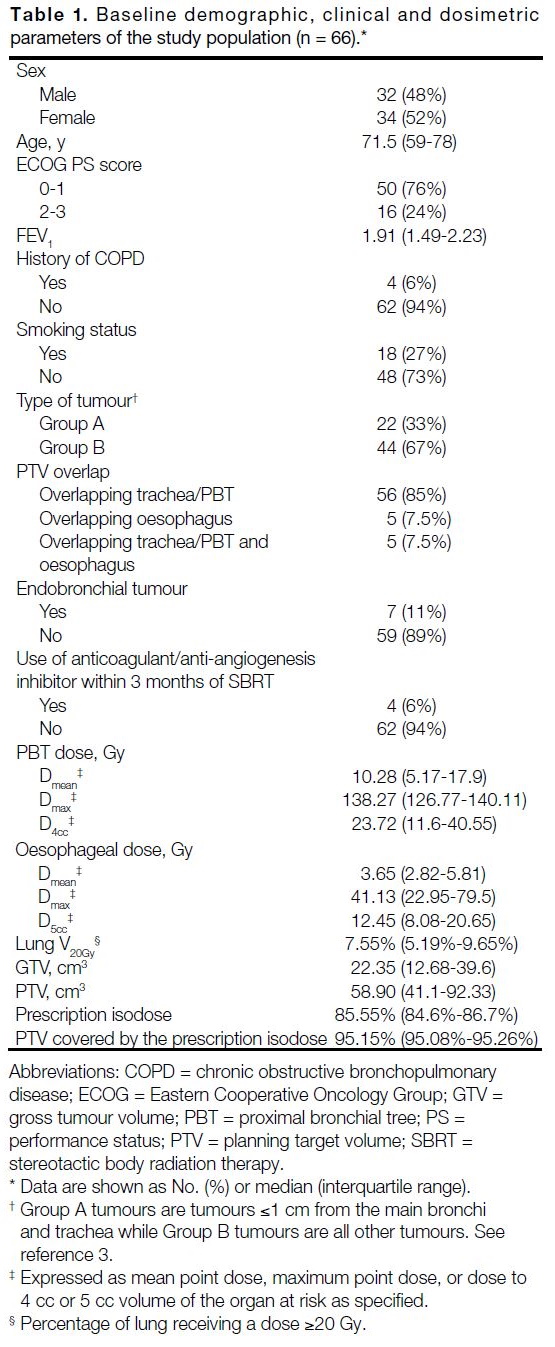
A total of 66 patients were analysed. The median follow-up was 54 months (range, 4-114). Baseline patient
characteristics and dosimetric parameters are detailed in
Table 1. The median age was 71.5 years. Most patients
(76%) had an Eastern Cooperative Oncology Group
performance status score of 0 to 1.
Table 1. Baseline demographic, clinical and dosimetric
parameters of the study population (n = 66).
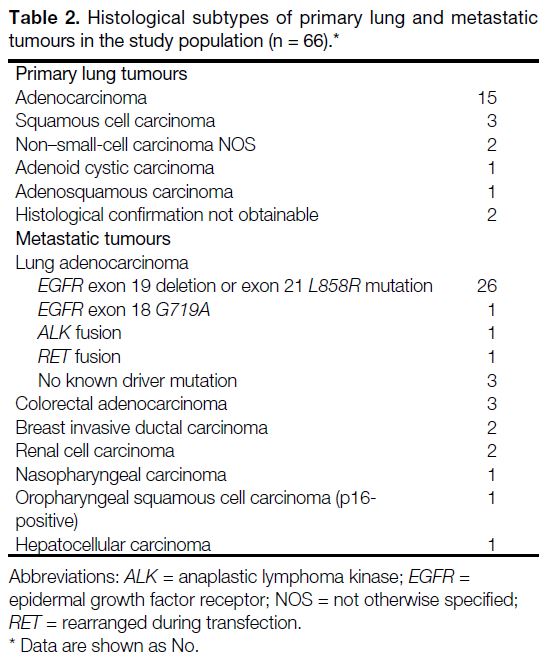
Twenty-four cases were primary lung tumours and 42
cases were lung metastases. The histological diagnoses
for primary lung and metastatic lesions are shown in
Table 2. Most metastatic lesions (32/42) were of lung origin. Indications of SBRT for lung metastases included
oligoprogression (n = 23), oligoresidual disease (n = 13),
and oligorecurrence (n = 6).
Table 2. Histological subtypes of primary lung and metastatic
tumours in the study population (n = 66).
Breath hold was used in 11 patients (17%), with the
remainder utilising 4D-CT simulation. The median
prescription isodose was 85.55%. Median GTV and PTV
were 22.35 cm3 and 58.90 cm3, respectively. Tumour
PTV overlapped with the PBT or trachea in 61 lesions
and oesophagus in 10 lesions. The median PTV coverage
by the prescription isodose was 95.15% (Table 1).
Local Control and Survival Outcomes
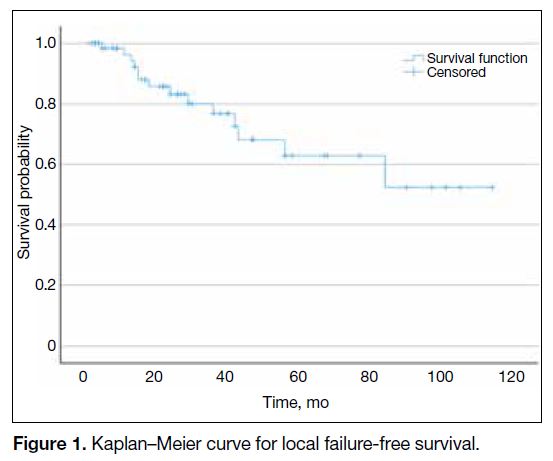
The 1-year and 3-year local failure-free survival rates
were 98% and 88%, respectively. Mean local failure-free
survival was 79 months (95% confidence interval
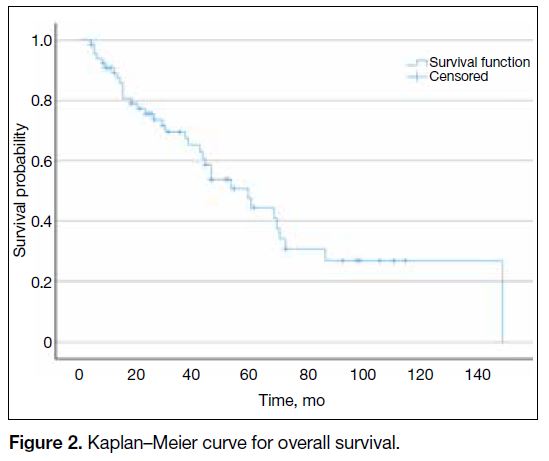
[CI]=65-94) [Figure 1]. OS ranged from 4 to 148
months, with a median OS of 59 months (95% CI = 54-85) [Figure 2]. The 1-year and 3-year OS rates were 89.4% and 69.7%, respectively.
Figure 1. Kaplan–Meier curve for local failure-free survival.
Figure 2. Kaplan–Meier curve for overall survival.
Safety Outcomes
Grade ≥2 toxicity occurred in 18 patients (27%). Three
patients (5%) had grade 3 toxicity, including oesophageal
stricture, radiation pneumonitis, and lung collapse for
each. Four patients (6%) had grade 5 toxicity (one case of oesophageal ulcer bleeding, two cases of airway
bleeding, and one case of multifactorial respiratory
failure). The median time to grade ≥3 toxicity was 4.5
months.
Among the 61 patients with PTV overlapping the PBT
or trachea, grade ≥2 pulmonary toxicity occurred in
14 cases (23%). Airway obstruction and/or bleeding
occurred in all 14 patients, and eight also had radiation
pneumonitis. Among the 10 patients with PTV
overlapping the oesophagus, four (40%) developed
grade ≥2 oesophageal toxicity, including two with
odynophagia, one with oesophageal stricture, and one
with oesophageal ulcer bleeding.
When comparing patients with or without grade ≥2
airway toxicity (bleeding or obstruction), there was a
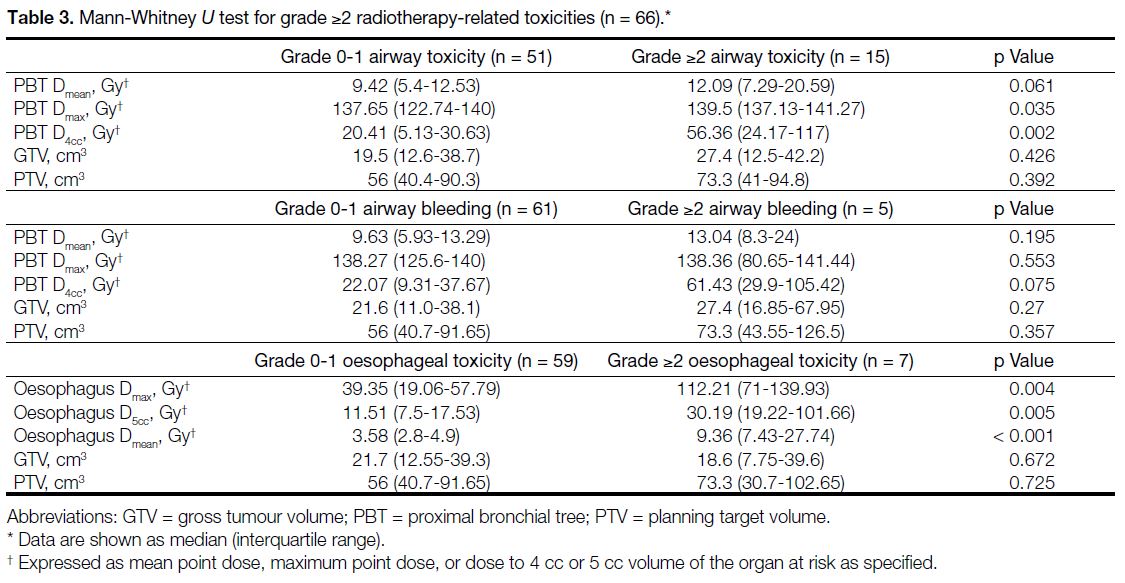
statistically significant difference for higher Dmax (p = 0.035) and higher dose to 4 cc (D4cc) of the PBT (p = 0.002) [Table 3].
Table 3. Mann-Whitney U test for grade ≥2 radiotherapy-related toxicities (n = 66).
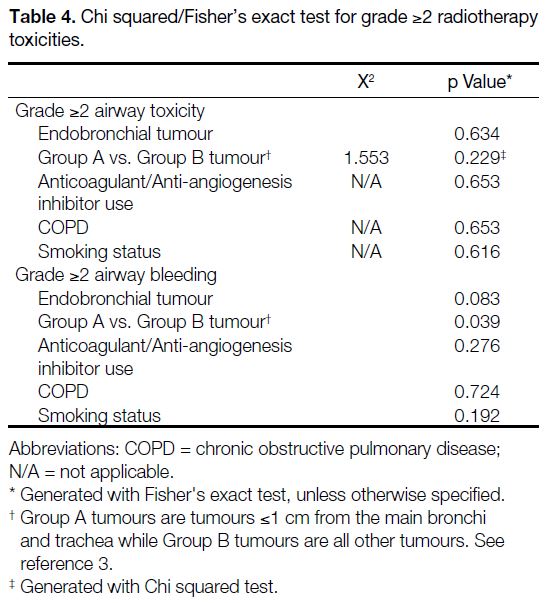
For grade ≥2 airway bleeding, a statistically significant
difference was found with group A tumours (≤1 cm
from the main bronchi and trachea)[3] [p = 0.039], while
a higher D4cc of the PBT (p = 0.075) and endobronchial
tumour location (p = 0.083) did not reach statistical
significance. The use of anticoagulant or antiangiogenic
therapy was not significantly associated with grade ≥2
bleeding (p = 0.276) [Table 4].
Table 4. Chi squared/Fisher’s exact test for grade ≥2 radiotherapy toxicities.
Baseline forced expiratory volume in 1 second, smoking
status, history of chronic obstructive pulmonary disease,
and the percentage of lung receiving a dose ≥20 Gy
were not significantly associated with grade ≥2 radiation
pneumonitis. For grade ≥2 oesophageal toxicity,
statistically significant differences were found in higher
mean dose (Dmean) [p < 0.001], higher Dmax (p = 0.004), and higher dose to 5 cc (D5cc) of the oesophagus (p = 0.005) [Table 3].
No postmortems were performed; thus, all deaths
classified as grade 5 events were based on clinical
grounds alone.
Simple and Multivariable Analyses
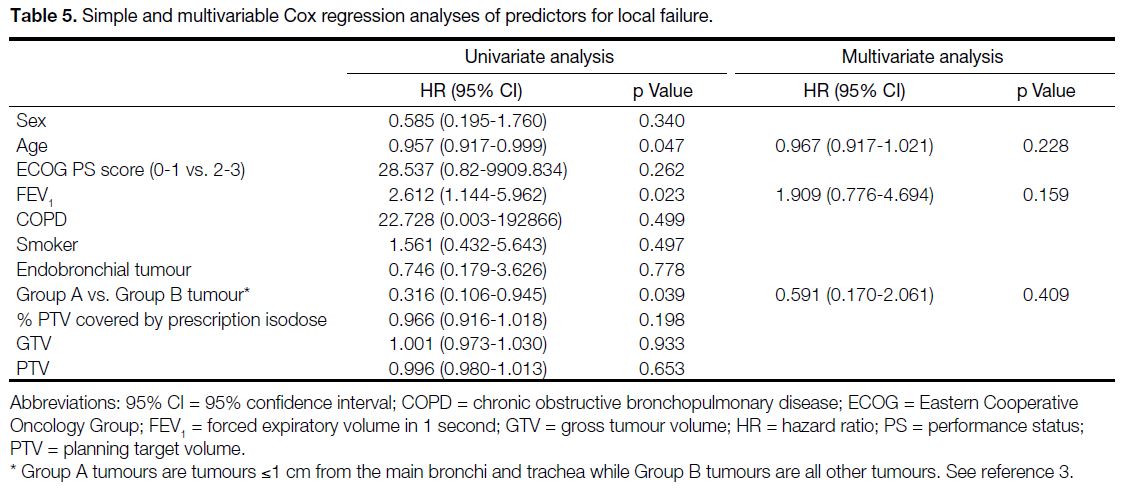
Simple Cox regression found age (hazard ratio [HR] = 0.957, 95% confidence interval [95% CI] = 0.917-0.999;
p = 0.047), and group A tumours (HR = 0.316, 95% CI = 0.106-0.945; p = 0.039) were predictors for local failure;
however, these variables were not significant in the
multivariable analysis (Table 5). Simple cox regression
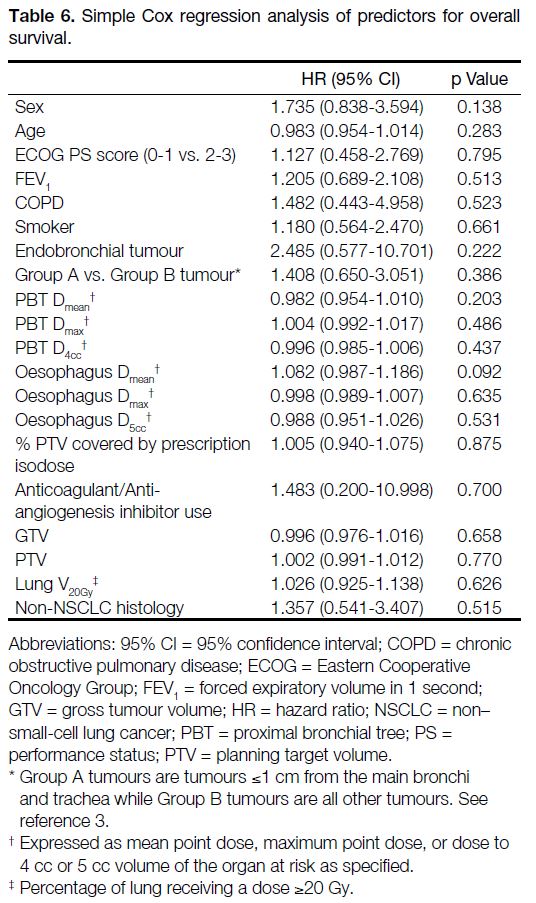
did not find any significant predictors for overall survival
(Table 6).
Table 5. Simple and multivariable Cox regression analyses of predictors for local failure.
Table 6. Simple Cox regression analysis of predictors for overall survival.
DISCUSSION
Our study provides some of the longest follow-up data
on the real-world outcomes of SBRT to ultra-central
lung tumours. With a median follow-up of 54 months,
our 3-year local control rate of 88% and grade 3 and
grade 5 toxicity rates (5% and 6%, respectively) were
comparable to prior studies.[6] In a recent meta-analysis
of ultra-central SBRT including 1183 patients over 27
studies,[6] the pooled 2-year local control rate was 89%,
while the grade 3 to 4 toxicity rate was 6% and the grade
5 toxicity rate was 4%.
A primary concern in ultra-central lung SBRT is
radiation-induced airway bleeding. In our study, grade
≥2 airway bleeding occurred in only five patients (8%),
including three fatal haemorrhages, representing 5%
of the study population. A possible reason may be our
institutional practice of limiting hotspots to 120%, in contrast to the HILUS trial[3] where hotspots of up to
150% were allowed.
Our univariate analysis revealed that grade ≥2 airway
toxicity was associated with a higher D4cc of the PBT,
and airway bleeding occurred more frequently in group
A tumours. This is consistent with findings of prior
dosimetric studies[7] [8] where the majority of fatal lung
haemorrhages were observed in group A tumours, with
rates of 70% to 89%.
Our grade 5 toxicity rate of 6% is comparable to previous
studies on ultra-central lung SBRT.[6] Among these,
two patients had bronchoscopy-proven endobronchial
tumour involvement. Although endobronchial tumour
location was more common in patients with grade ≥2
airway bleeding the association did not reach statistical
significance (p = 0.083) [Table 4]. This parallels findings
from Tekatli et al[9] where endobronchial tumours
comprised 46% of all SBRT-related grade ≥3 lung
haemorrhages.
In our cohort, 10 patients had the PTV overlapping the
oesophagus, and grade 3 to 5 events occurred in three
of them. Literature focusing specifically on oesophageal
toxicity in SBRT is limited, with small sample size. In
Wang et al’s retrospective study[10] of 88 patients, 23 tumours had the PTV overlapping the oesophagus.
Grade ≥3 oesophageal toxicity rate was 13%, including two cases of tracheoesophageal fistulisation.[10]
Univariate analysis suggested that shorter distance
between the tumour and the oesophagus predicted
toxicity and suggested the use of more protracted
fractionation.[10]
Our dosimetric analysis revealed that oesophageal Dmax, D5cc, and Dmean were associated with higher rates of grade ≥2 oesophageal toxicity. However, the optimal threshold
for oesophageal toxicity remains undefined in the
literature. Among the 10 patients whose PTV overlapped
the oesophagus, D5cc exceeded the RTOG 0813 constraint
of 27.5 Gy in three patients, two of whom had grade ≥3
events. This suggests that strict adherence to a D5cc of
<27.5 Gy may help to reduce severe toxicity.
Taken together, our results and the literature suggest
that lesions close to/abutting the oesophagus carry a
substantial risk of SBRT-related toxicity. Protracted
fractionations and avoiding tumours with direct invasion
or abutment of the oesophagus would be advisable to
reduce severe toxicity. Further data are awaited to define
optimal dose constraints and fractionation for these
tumours.
Limitations
Our study had several limitations, including its
retrospective nature and non-randomised design. Our
sample size was also small, and the number of events
was too limited for detailed statistical analyses and
elucidation of safe dose constraints for the investigated toxicity endpoints. Our database relied on clinical
records documented by treating clinicians rather than a
prospective database for research purposes; thus, some
toxicities may have been underreported. The lack of
autopsy information on the exact cause of death also
made it difficult to definitively conclude whether they
were truly SBRT-related mortalities.
CONCLUSION
In our study of 66 patients undergoing ultra-central
SBRT, long-term follow-up showed sustained high
rates of local control and acceptable toxicity outcomes.
Caution should be taken when delivering SBRT to group
A lesions, and attention should be paid to dosimetric
constraints such as the D4cc of the PBT and the D5cc of
the oesophagus. Further studies are needed to clarify the
optimal dose fractionation and organ at risk constraints
to minimise toxicity.
REFERENCES
1. Tateishi Y, Takeda A, Horita N, Tsurugai Y, Eriguchi T, Kibe Y,
et al. Stereotactic body radiation therapy with a high maximum dose
improves local control, cancer-specific death, and overall survival
in peripheral early-stage non–small cell lung cancer. Int J Radiat
Oncol Biol Phys. 2021;111:143-51. Crossref
2. Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL,
Ryan WF, et al. Safety and efficacy of a five-fraction stereotactic
body radiotherapy schedule for centrally located non–small-cell
lung cancer: NRG Oncology/RTOG 0813 trial. J Clin Oncol.
2019;37:1316-25. Crossref
3. Lindberg K, Grozman V, Karlsson K, Lindberg S, Lax I, Wersäll P,
et al. The HILUS-trial—a prospective Nordic multicenter phase 2 study of ultracentral lung tumors treated with stereotactic body
radiotherapy. J Thorac Oncol. 2021;16:1200-10. Crossref
4. Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W,
Kavanagh B, et al. Stereotactic body radiation therapy: the report
of AAPM Task Group 101. Med Phys. 2010;37:4078-101. Crossref
5. United States Department of Health and Human Services.
Common Terminology Criteria for Adverse Events (CTCAE)
Version 5.0. 2017 Nov 27. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 30 Apr 2025.
6. Yan M, Louie AV, Kotecha R, Ashfaq Ahmed M, Zhang Z, Guckenberger M, et al. Stereotactic body radiotherapy for ultra-central lung tumors: a systematic review and meta-analysis and
International Stereotactic Radiosurgery Society practice guidelines.
Lung Cancer. 2023;182:107281. Crossref
7. Tekatli H, Giraud N, van Eekelen R, Lagerwaard FJ, Senan S. Ten
years outcomes after SABR in central and ultracentral primary lung
tumors. Radiother Oncol. 2023;188:109848. Crossref
8. Lindberg S, Grozman V, Karlsson K, Onjukka E, Lindbäck E,
Jirf KA, et al. Expanded HILUS trial: a pooled analysis of risk
factors for toxicity from stereotactic body radiation therapy of
central and ultracentral lung tumors. Int J Radiat Oncol Biol Phys.
2023;117:1222-31. Crossref
9. Tekatli H, Duijm M, Oomen-de Hoop E, Verbakel W,
Schillemans W, Slotman BJ, et al. Normal tissue complication
probability modeling of pulmonary toxicity after stereotactic and
hypofractionated radiation therapy for central lung tumors. Int J
Radiat Oncol Biol Phys. 2018;100:738-47. Crossref
10. Wang C, Rimner A, Gelblum DY, Dick-Godfrey R, McKnight D, Torres D, et al. Analysis of pneumonitis and esophageal injury after
stereotactic body radiation therapy for ultra-central lung tumors.
Lung Cancer. 2020;147:45-8. Crossref