Outcomes of Patients with Unresectable Stage III Non–Small-Cell Lung Cancer Treated with Durvalumab After Chemoradiotherapy
ORIGINAL ARTICLE
Hong Kong J Radiol 2025;28:Epub 12 June 2025
Outcomes of Patients with Unresectable Stage III Non–Small-Cell Lung Cancer Treated with Durvalumab After Chemoradiotherapy
SSN Leung, MY Lim, TTS Lau
Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
Correspondence: Dr SSN Leung, Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China. Email: sheonaleung@ha.org.hk
Submitted: 23 August 2024; Accepted: 25 November 2024. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. SSNL acquired the data. SSNL and MYL analysed the data. SSNL drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-185-2). The requirement for informed patient consent was waived by the Board due to the retrospective nature of the research and the use of anonymised data in the research.
Abstract
Introduction
This study evaluated the efficacy and safety of durvalumab in unresectable stage III non–small-cell
lung carcinoma (NSCLC) at a tertiary centre in Hong Kong.
Methods
Cases of stage III NSCLC treated with radical-intent chemoradiotherapy (CRT), with or without durvalumab, from December 2017 to June 2023 were included. Outcomes, including progression-free survival (PFS) and overall survival (OS), were analysed using the Kaplan–Meier method. Adverse events, including any-grade pneumonitis and the Common Terminology Criteria for Adverse Events grade ≥3 immune-related adverse events, were reviewed.
Results
A total of 113 cases were analysed (51 cases of durvalumab plus CRT and 62 cases of CRT). The durvalumab
plus CRT cohort demonstrated a significantly longer median PFS compared to the CRT cohort (34.9 vs. 10.5 months; p = 0.01), while median OS remained immature at the time of analysis. Among patients with epidermal growth factor receptor (EGFR) mutations, the estimated PFS also favoured the durvalumab plus CRT cohort. A significantly higher incidence of any-grade pneumonitis was observed in the durvalumab plus CRT cohort (31% vs. 8%; p = 0.002), with most cases occurring within the initial 3 months of durvalumab use.
Conclusion
Durvalumab following CRT significantly benefitted patients with unresectable stage III NSCLC, including those with EGFR mutations. Symptomatic pneumonitis tended to occur in the first 3 months of durvalumab therapy and was generally manageable. Closer follow-up during this period is recommended to facilitate early detection and intervention. Further research is warranted to understand the complex interplay among EGFR mutation status, programmed death ligand 1 expression, and treatment outcomes with and without durvalumab in NSCLC.
Key Words: Carcinoma, non–small-cell lung; Chemoradiotherapy; ErbB receptors; Progression-free survival
中文摘要
無法切除的第三期非小細胞肺癌患者在同步化學放射治療後接受度伐魯單抗治療的療效分析
梁詩雅、林美瑩、劉芷珊
引言
本研究旨在評估香港一所三級醫院針對無法切除的第三期非小細胞肺癌患者採用度伐魯單抗作為鞏固治療的效益及安全性。
方法
本研究回溯性納入於2017年12月至2023年6月期間接受根治性同步化學放射治療(放化療)的無法切除第三期非小細胞肺癌患者,依據後續是否接受度伐魯單抗治療分組。我們使用Kaplan-Meier法分析疾病無惡化存活期及整體存活期,並系統性評估不良事件,涵蓋各級別非感染性肺炎及符合「常見不良事件評價標準」【CTCAE】第3級或以上免疫相關不良反應。
結果
本研究共分析了113例患者,包括51例放化療合併度伐魯單抗及62例僅接受放化療。放化療合併度伐魯單抗組的疾病無惡化存活期中位數顯著較單獨放化療組長(34.9個月與10.5個月;p = 0.01),整體存活期中位數則在分析時尚未成熟。在表皮生長因子受體(EGFR)基因突變患者中,放化療合併度伐魯單抗組也呈現較長的預估疾病無惡化存活期。安全性方面,放化療合併度伐魯單抗組在非感染性肺炎總發生率顯著較高(31%與8%;p = 0.002),且多數病例集中於治療起始3個月內發生。
結論
同步放化療後接續度伐魯單抗治療對於第三期非小細胞肺癌患者(包括EGFR基因突變患者)具顯著臨床效益。症狀性肺炎雖易於治療初期首3個月出現,但整體可控。我們建議在此段期間密集隨訪,以監察非感染性肺炎的早期徵狀。EGFR基因突變狀態、細胞程式死亡─配體1(PD-L1)表現量及度伐魯單抗的治療效益存在複雜相互作用,有待未來研究作進一步釐清。
INTRODUCTION
The PACIFIC trial[1] showed that 1 year of durvalumab
consolidation therapy following chemoradiotherapy
(CRT) significantly improves the progression-free
survival (PFS) and overall survival (OS) in unresectable
stage III non–small-cell lung cancer (NSCLC), with a
median PFS of 16.9 months and OS of 47.5 months.[2]
The PACIFIC-R study[3] substantiated these findings,
suggesting that real-world outcomes align with the
drug’s registration trial results.[4]
In Hong Kong, durvalumab has been a registered drug
since October 2018 and included in the Community Care
Fund Medical Assistance Programme since May 2020.[5]
Given the emerging concern that patients harbouring
epidermal growth factor receptor (EGFR) mutations may
derive less benefit from immune checkpoint inhibitors,
including maintenance durvalumab, studies have been
conducted to review the outcomes in this subgroup.[6] [7] [8]
Pneumonitis, a major adverse event associated with durvalumab, is of particular concern in patients who
have undergone thoracic radiotherapy.
This study aimed to evaluate the real-world efficacy and
safety of durvalumab in unresectable stage III NSCLC in
a population with a high prevalence of EGFR mutations
and to assess pneumonitis incidence relative to radiation
dose, enabling early toxicity detection and optimising
follow-up protocols to ensure that local patients achieve
maximal therapeutic benefit with minimised risks.
METHODS
Inclusion Criteria and Data Collection
This retrospective study included patients with stage
III NSCLC who were treated with chemoradiotherapy
(CRT) between December 2017 and June 2023
in Princess Margaret Hospital, Hong Kong. The
durvalumab cohort was drawn from the Clinical Data
Analysis and Reporting System of Hospital Authority,
comprising all patients who received durvalumab during the specified period. The CRT cohort—patients who
received CRT only—was drawn from our department’s
ARIA Oncology Information System. Each case was
screened via the Electronic Patient Record system for
eligibility. Inclusion criteria were adult patients aged
≥18 years, diagnosed with stage III NSCLC and treated
with CRT with curative intent. All patients were restaged
using the 8th edition of the American Joint Commission
on Cancer TNM (tumour, node and metastasis)
Classification.[9] Patients who had commenced treatment
in other centres must have received at least one dose of
durvalumab in our hospital to be included in the analysis.
Cases of proven disease progression within 2 months
of CRT completion were excluded. Patient and disease
demographics, details of chemoradiotherapy treatment
regimens, and response to treatment were documented.
Treatment-related toxicities were graded according to
the CTCAE (Common Terminology Criteria for Adverse
Events) version 5.0.[10]
Treatment and Follow-up
Standard radical-intent CRT in the stage III NSCLC
study population involved three-dimensional conformal
radiotherapy of 60 to 66 Gy at 2 Gy per fraction, typically
paired with etoposide/cisplatin for two cycles once
every 3 weeks. For non-squamous cases, pemetrexed/cisplatin was an alternative, especially for patients with
poor venous access or concerns about tolerance. Patients
unsuitable for cisplatin (e.g., creatinine clearance
<50 mL/min or congestive heart failure) received
weekly paclitaxel/carboplatin. Induction chemotherapy
was planned on a case-by-case basis. Optimal organs-at-risk dose constraints were: (1) the percentage of lung
receiving ≥20 Gy (lung V20Gy) ≤30%; (2) lung V5Gy
≤55%; and (3) mean lung dose (MLD) ≤15 Gy.
Durvalumab consolidation was offered to eligible
patients without progression after CRT as self-funded
treatment since October 2018, or with financial
assistance from the Community Care Fund for those
with programmed death ligand 1 (PD-L1) expression
of tumour proportion score ≥1% since May 2020.[5]
Durvalumab at 10 mg/kg biweekly for up to 12 months
was usually started within 42 days post-radiotherapy,
though this was not mandatory. Pre-cycle chest
radiographs (CXR) and laboratory tests, including
complete blood count, liver/renal/thyroid function,
cortisol level, and fasting glucose level were taken to
monitor for adverse events. Post-treatment, patients
were followed up every 4 to 6 months with CXR, and
carcinoembryonic antigen was also measured in cases of adenocarcinoma. Computed tomography scans were
performed subject to availability and clinical judgement.
Statistical Analyses
Baseline characteristics and dosimetric parameters of
the two cohorts were compared using Chi squared or
Fisher’s exact tests. PFS and OS were measured from
the last day of radiotherapy to disease progression or
death. The data cut-off was 15 June 2024. The Kaplan-Meier method was utilised to estimate PFS and OS.
Subgroup analysis explored outcomes in EGFR-mutated
(EGFRm) and EGFR–wild-type (EGFRwt) patients. Univariate logistic regression analysis was employed
to evaluate any significant predictive factors (clinical or
dosimetric) for the incidence of any-grade pneumonitis,
with only significant univariate factors further analysed
by multivariate analysis. Statistical analyses were
conducted using commercial software SPSS (Windows
version 29.0; IBM Corp, Armonk [NY], US), each with
a significance level of 0.05. For missing data, a listwise
deletion approach was employed to analyse cases with
complete data only. Receiver operating characteristic
(ROC) analysis was conducted as an exploratory
measure to identify an optimal cut-off value for lung
V20Gy associated with pneumonitis occurrence.
RESULTS
Patient Characteristics
This study included 113 cases, with 51 in the durvalumab
plus CRT cohort and 62 in the CRT cohort (Table 1).
Both cohorts had a predominance of male and smoker/ex-smoker patients. The median ages were 65 years
and 66.5 years in the durvalumab plus CRT and CRT
cohorts, respectively. Baseline characteristics were
similar, except for a higher proportion of patients with
no PD-L1 expression in the CRT cohort compared with
the durvalumab plus CRT cohort. Histology was mainly
adenocarcinoma (41% in the durvalumab plus CRT
cohort and 48% in the CRT cohort) and squamous cell
carcinoma (27% and 37%, respectively). NSCLC of no
specific type was reported in 27% of the durvalumab plus
CRT cohort and 6% of the CRT cohort. Approximately
70% of patients had their EGFR status tested; 7 (14%)
and 15 (24%) patients in the durvalumab plus CRT
and CRT cohorts, respectively, were confirmed as
EGFRm. Commonly used CRT chemotherapy regimens
were etoposide/platinum (37% and 48%), paclitaxel/carboplatin (35% and 37%), and pemetrexed/platinum
(12% and 10%) in the durvalumab plus CRT and
CRT cohorts, respectively. The median duration from
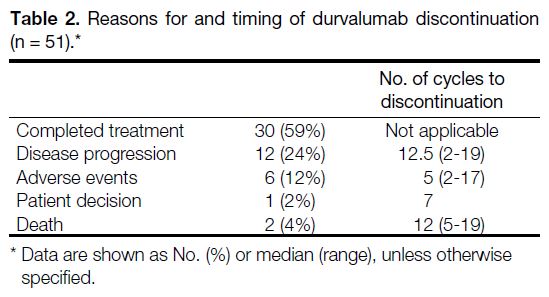
CRT completion to durvalumab initiation was 45 days (range, 8-172); 59% (n = 30) of patients completed the
planned 26 cycles of biweekly durvalumab (median:
13.8 months). Treatment discontinuation was attributed
to disease progression, adverse events, patient decision,
or death (Table 2).
Table 1. Baseline characteristics (n = 113).
Table 2. Reasons for and timing of durvalumab discontinuation (n = 51).
At the time of analysis, all patients in the durvalumab
plus CRT cohort had either discontinued or completed
26 cycles of durvalumab consolidation treatment. 94%
and 95% patients in the durvalumab plus CRT and CRT
cohorts, respectively, had completed CRT, defined as
either having received chemotherapy once every 3 weeks
for 2 cycles or a concurrent regimen once a week for 5
cycles. All patients, except for one treated in the private
sector with missing data, received a radical dose of at
least 60 Gy (equivalent dose in 2 Gy fractions).
Efficacy Outcomes
The median follow-up was 25.6 months for the
durvalumab plus CRT cohort and 31.0 months for the
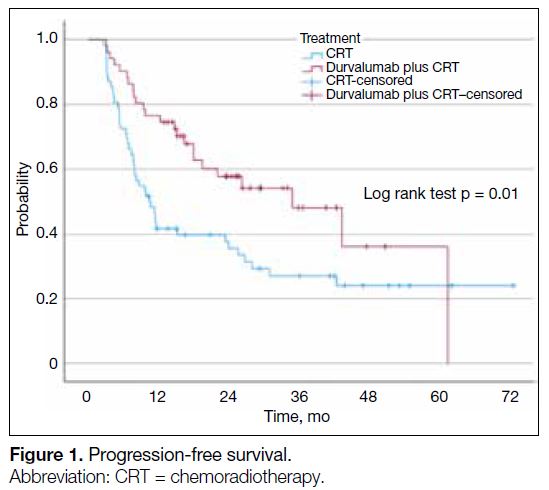
CRT cohort. The median PFS was significantly longer in
the durvalumab plus CRT cohort, at 34.9 months (95%
confidence interval [CI] = 17.8-52.0) compared to 10.5
months (95% CI = 7.1-14.0) in the CRT cohort (p = 0.01)
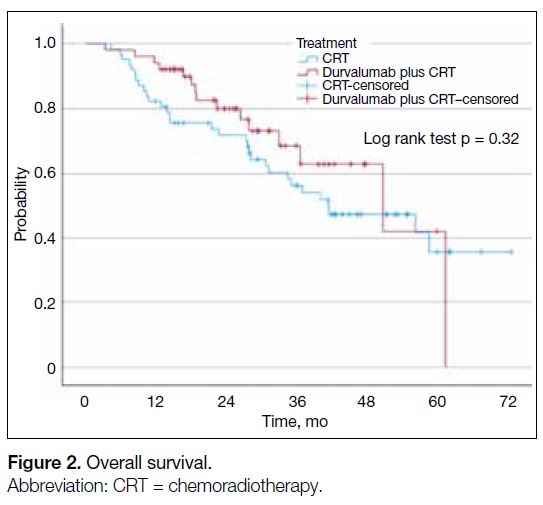
[Figure 1]. The median OS was 50.8 months (95% CI = 26.6-75.0) in the durvalumab plus CRT cohort and 41.5
months (95% CI = 22.2-60.7) in the CRT cohort, which
was not statistically significant (p = 0.32) [Figure 2].
Figure 1. Progression-free survival.
Figure 2. Overall survival.
The estimated PFS for EGFRm patients was not reached in the durvalumab plus CRT cohort, compared to 7.8
months (95% CI = 3.4-12.1) in the CRT cohort. OS
analysis was not performed due to the limited number
of events (one in the durvalumab plus CRT cohort and
8 in the CRT cohort). Notably, all EGFRm patients in
the durvalumab plus CRT cohort had either unknown or
low PD-L1 expression, while those in the CRT cohort
had either unknown or negative PD-L1 expression. No
EGFRm patients had high PD-L1 expression.
Pneumonitis
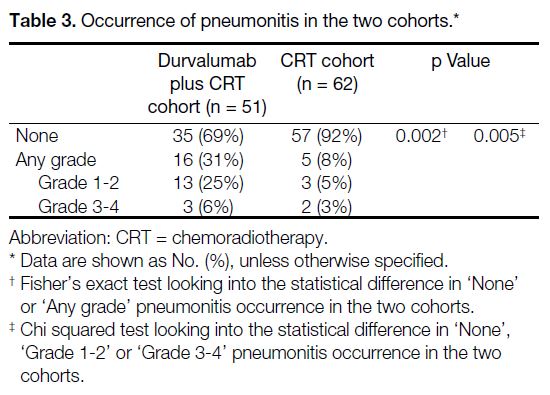
A significantly higher incidence of any-grade
pneumonitis was observed in the durvalumab plus
CRT cohort compared to the CRT cohort (31% vs. 8%;
p = 0.002) [Table 3]. In total, 57% of EGFRm patients
and 27% of EGFRwt/EGFR-unknown patients in the
durvalumab plus CRT cohort developed pneumonitis,
compared to 0% and 10%, respectively, in the CRT
cohort. Of the 16 patients in the durvalumab plus
CRT cohort who developed pneumonitis, the majority
(87.5%) experienced their first episode during the initial
six biweekly cycles (range, 2-12). Approximately 80%
of cases were grade 1 to 2 and responded to appropriate management strategies including corticosteroids, except
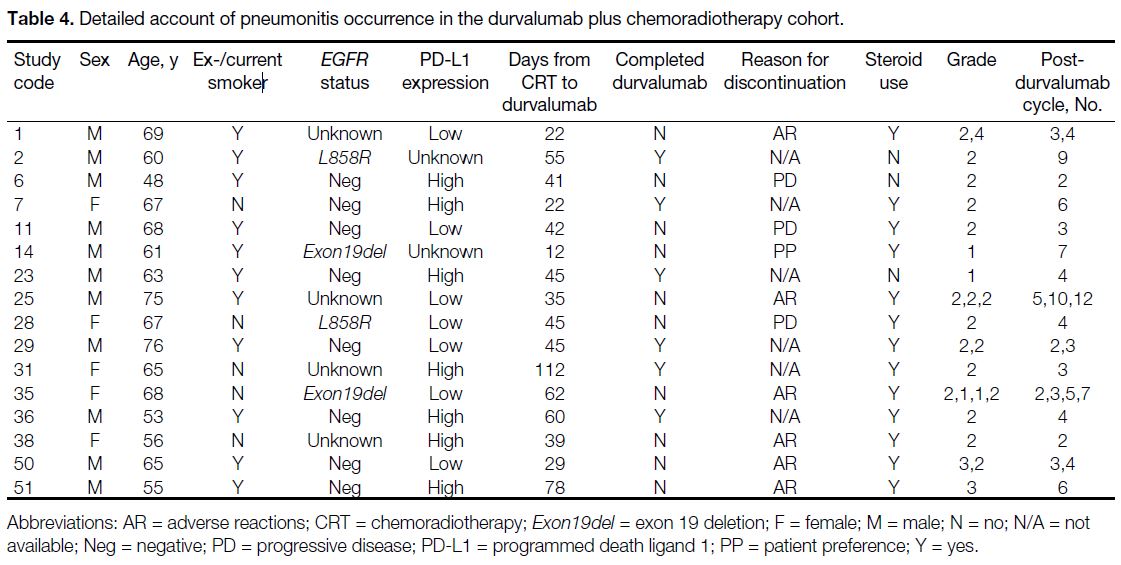
one grade 4 pneumonitis (Table 4). Overall, 12%
discontinued durvalumab treatment due to pneumonitis.
In the CRT cohort, five patients (8%) developed any
grade of radiation pneumonitis (RP), with onset ranging
from 6 to 91 days after the last day of radiotherapy. All
improved clinically after a course of steroids.
Table 3. Occurrence of pneumonitis in the two cohorts.
Table 4. Detailed account of pneumonitis occurrence in the durvalumab plus chemoradiotherapy cohort.
The single case of grade 4 pneumonitis in the
durvalumab plus CRT cohort was a patient with a history
of rectal and hepatocellular carcinoma in remission,
who was diagnosed with a third primary, T4N0
poorly differentiated NSCLC with focal squamous
differentiation. The patient received two cycles of
induction 3-weekly paclitaxel/carboplatin followed by CRT and subsequently three weekly cycles of paclitaxel/carboplatin due to neutropenia and thrombocytopenia. A
computed tomography scan performed 1 day after CRT
completion showed stable disease, leading to durvalumab
initiation on day 22. He developed grade 2 pneumonitis
before cycle 4 of durvalumab, leading to treatment
suspension and initiation of a 1-month tapering course of
prednisolone at 1 mg/kg. After radiological and clinical
improvement, cycle 4 of durvalumab was resumed 43
days after its original planned date. Seven days later,
he was admitted for respiratory failure requiring high-flow
oxygen. Intravenous methylprednisolone 2 mg/kg
was administered for 5 days, but there was further
consolidation on CXR treated with one dose of infliximab
on day 6. He was subsequently transitioned to oral
prednisolone on day 54, with clinical improvement and
reduced oxygen requirement. He became deconditioned
3 months later after steroid weaning, developing brain
metastases and hospital-acquired pneumonia, and
succumbed after 140 days of hospitalisation.
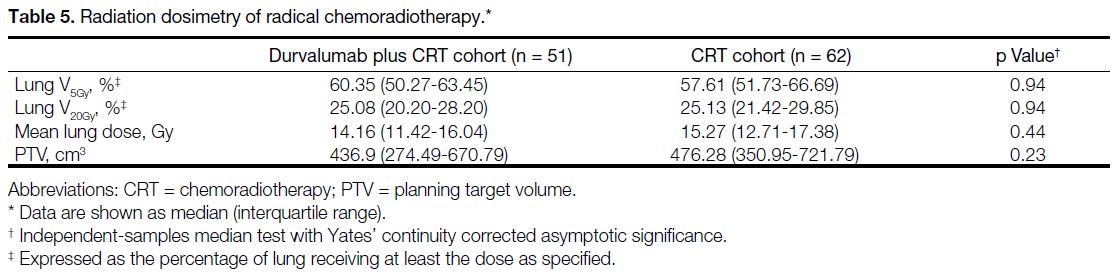
No significant differences were observed in lung V5Gy,
lung V20Gy, MLD, or planning target volume between the
two cohorts (Table 5). Among these parameters, only
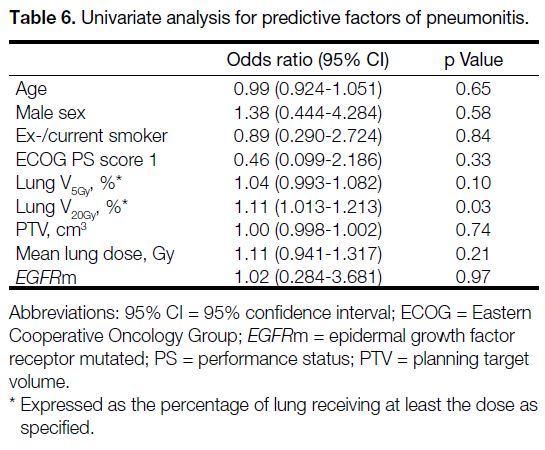
lung V20Gy demonstrated a significant correlation with any
grade pneumonitis in univariate logistic analysis, with
an odds ratio of 1.11 (95% CI = 1.013-1.213; p = 0.03),
indicating that for each 1% increase in the volume of lung
receiving ≥20 Gy, the odds of developing pneumonitis
increased by approximately 11% (Table 6). Focusing on the durvalumab plus CRT cohort, ROC analysis
identified an optimal lung V20Gy threshold of 22.76% for
predicting pneumonitis, with a Youden’s index of 0.469,
optimising sensitivity (0.92) and specificity (0.46). The
area under the curve of the ROC analysis was 0.71,
indicating moderate discriminatory power.
Table 5. Radiation dosimetry of radical chemoradiotherapy.
Table 6. Univariate analysis for predictive factors of pneumonitis.
Grade 3 or 4 Immunotherapy-Related Adverse Events Within the Durvalumab Cohort
The overall incidence of grade 3 or 4 immune-related
adverse events was 13.7% (7/51). Three patients (6%)
had RP, with one concurrently developing grade 3
hepatitis after 6 cycles that resolved over 2 months of
corticosteroid treatment. For the remaining four patients,
two (3.9%) developed grade 3 hyperglycaemia without
a baseline history of diabetes, one (2%) experienced
grade 3 skin rash after 17 cycles of durvalumab, and
one (2%) developed grade 3 pneumonia. No patients
discontinued durvalumab due to adverse events other
than pneumonitis.
DISCUSSION
Our durvalumab plus CRT cohort demonstrated superior
PFS to the CRT cohort, consistent with findings from the
PACIFIC trial and real-world studies.[2] [11] [12] [13] The disparity
in median follow-up times between the CRT cohort (31
months) and the durvalumab plus CRT cohort (25.6
months) may be attributed to delayed availability of
durvalumab funding, resulting in more patients receiving
CRT alone between 2018 to 2020. This complicates PFS
and OS interpretation, especially with the survival curve
of the durvalumab plus CRT cohort plateauing.
Our mean PFS durations of 34.9 months (the
durvalumab plus CRT cohort) and 10.5 months (the
CRT cohort) exceeded that of the PACIFIC trial results,[2]
nearly doubling their reported numbers. While real-world
follow-up variability might underestimate early
progression, prognostic advantages in our cohort likely
contributed. These included a higher proportion with
Eastern Cooperative Oncology Group performance
status score of 0 (88% vs. 50% in the PACIFIC
trial[14]) and more never-smokers (25% vs. 9%). PD-L1
status showed dual roles: in the CRT cohort, the
higher proportion of PD-L1-negative patients (~50%)
aligns with its known favourable prognostic value in
the pre-immunotherapy era, supported by multiple
meta-analyses.[15] [16] [17] [18] Conversely, the PD-L1–enriched
population in the durvalumab plus CRT cohort (~90%
positive, ~50% with ≥ 50% expression) reflect its
predictive value, consistent with the PACIFIC subgroup
analysis showing enhanced immunotherapy benefit with
higher PD-L1 expression.[2] Additionally, approximately
half of the cohort received at least one cycle of induction
chemotherapy, compared to only a quarter in the
PACIFIC trial.[2] Any potentiation of immunotherapy
with induction chemotherapy, through neoantigen
release and tumour microenvironment modulation, is a
theoretical consideration. Further elucidation, however,
is required to determine the application of PD-L1 for risk stratification and to optimise treatment sequencing and
combination, including toxicity risks.[19] [20]
The incidence of any-grade pneumonitis in our
durvalumab plus CRT cohort (31%) was similar to the
figure reported in the PACIFIC study (34%),[2] where it
was the most common adverse event leading to treatment
discontinuation (6.3%).[2] It was higher than in the CRT
cohort (8%), though the majority (~80%) were grade 1
to 2 per the CTCAE version 5.0 criteria.[10] Differentiating
between immunotherapy-induced pneumonitis (IP) and
RP, especially in the early cycles, proved challenging.
Radiologically, RP is more likely if the consolidative
changes are seen only within the irradiated field.
Observation from our study reinforced this diagnostic
difficulty as the majority of events occurred within the
first 3 months in both groups (87.5% in the durvalumab
plus CRT cohort vs. all within 91 days in the CRT
cohort). This aligns with other studies reporting median
pneumonitis onset around 3 to 4 months,[21] [22] emphasising
the importance of close monitoring during early
durvalumab treatment.
Fortunately, treatment is mostly similar for both
conditions with corticosteroids as the mainstay, although
IP may require longer treatment. In cases of steroid-refractory
IP, immunosuppressive agents such as
mycophenolate mofetil or infliximab can be considered.[23]
Supportive management such as symptom-relieving
medications and oxygen support should always be given
where clinically indicated. Vigilance for concomitant
infection due to the immunosuppressive effects of the
cancer treatments and high-dose steroids is also essential.
The decision to rechallenge with durvalumab after
resolution of low-grade pneumonitis should be made
after ensuring patients are well informed of recurrent
or higher-grade pneumonitis risks. Among patients
in the durvalumab plus CRT cohort who developed
pneumonitis, 31% experienced recurrence of events
after treatment resumption. Overall, 12% discontinued
durvalumab due to pneumonitis, similar to the reported
9.5% in the PACIFIC-R study.[4]
There is no doubt that RP could compromise patients’
outcomes and quality of life, therefore continuous efforts
have been put to identify any clinical and dosimetric
factors that are predictive and/or preventive. Lung
V20Gy is the most representative among the commonly
reported parameters. However, it is uncertain whether
the traditional dose constraints used in CRT are equally
applicable to patients also receiving immunotherapy. In our cohort, lung V20Gy was the only radiation dose
parameter that correlated with pneumonitis, with an
optimal threshold at 22.76% based on ROC analysis.
However, the low specificity (0.46) suggests that lung
V20Gy alone is not a strong predictor due to its high false
positive rate. Of note, this threshold is lower than the
commonly reported 30% for normofractionated thoracic
radiotherapy in the preimmunotherapy era. Even lower
thresholds, such as 18.77% in a Japanese study[21] and
15.8% in the Mayo Clinic, have been proposed for
predicting grade≥2 pneumonitis.[22] All these highlight a
change in regulation of immune and/or lung homeostasis
after exposure to immunotherapy and radiotherapy;
this could possibly lead to different lung parenchymal
susceptibilities. The high incidence of any-grade (88%)
and grade ≥3 pneumonitis (12%) in the Japanese study
involving 91 patients,[21] and Asian predominance in
pneumonitis after CRT with or without immunotherapy
in a recent meta-analysis over 20,000 patients[24] and in
the PACIFIC subgroup analysis[25] raise further research
questions with regard to any ethnic and/or genetic
contributing factors. Although direct comparison across
trials to derive the optimal dose constraint is not possible
due to varying radiotherapy planning techniques,
chemotherapy regimens, and patient factors, efforts to
reduce the lung V20Gy to as low as possible are reasonable.
Practically, applying more stringent lung dose constraints while maintaining target coverage in radiotherapy
planning for stage III NSCLC, where tumours are
often bulky, is challenging. Advanced technology,
including intensity-modulated radiation therapy and
proton therapy, may offer benefits over conventional
techniques.[26] However, uncertainty remains regarding
any interplay between low radiation exposure (e.g.,
lung V5Gy and MLD) and immunotherapy in modulating
pneumonitis risk. Moreover, the labour-intensive
nature of planning and treatment delivery warrants
careful patient selection, especially in high-workload or
resource-limited settings.
In addition to pneumonitis, our study also examined
all-cause immune-related grade 3 or 4 adverse events.
The incidence in our cohort (13.7% grade 3 and 3%
grade 4) were higher than in the PACIFIC trial (3.4%
in the durvalumab plus CRT cohort and 2.6% in the
CRT group),[2] but a solid conclusion on differences in
safety cannot be made due to the small sample size and
variable documentation of our study. Reassuringly,
a similar proportion of patients required durvalumab
discontinuation due to adverse events (12% in our study vs. 15.4% in the PACIFIC trial).[2] This underpins the
fact that adequate patient education together with team-based
engagement remain the key to ensuring timely
recognition and effective management of immune-related
adverse events.
When focusing on EGFRm patients, the estimated
PFS was not reached in the durvalumab plus CRT
cohort, compared with 7.8 months in the CRT cohort,
suggesting a potential benefit of adjuvant durvalumab.
This contrasts with the lack of benefit in the post-hoc
analysis of EGFRm subgroups in the PACIFIC trial[6]
and another retrospective review involving multiple
academic medical centres in the US.[7] However, caution
should be exercised when interpreting these results due
to the small sample size, the low treatment completion
rates (15%-50%) reported in the abovementioned
studies, and the short follow-up interval of our study.
Another notable observation from our durvalumab plus
CRT cohort is the higher occurrence of pneumonitis in
EGFRm patients (57%) compared to EGFRwt/unknown
patients (27%), though the difference was not
statistically significant. While the exact mechanism
underlying this difference remains unknown, this
observation carries important clinical implications as
initiating EGFR tyrosine kinase inhibitors (TKIs) after
CRT is a relatively common post-radical treatment
for EGFRm stage III disease due to the high risk of
progression. There is already growing recognition
of the increased risk of pneumonitis with sequential
immunotherapy followed by early TKI treatment.[27]
Prior RP and IP may exacerbate this risk through
increased lung tissue sensitivity, cumulative lung
injuries, and/or shared mechanisms such as immune
response dysregulation. Although none of our three
EGFRm patients who received erlotinib immediately
upon disease progression during durvalumab plus CRT
treatment developed pneumonitis, this should not over-reassure
clinicians given the safety alert reported in
other studies[28] [29] when using immunotherapy and TKIs
in close intervals. Optimal timing to guide safe use of
immunotherapy and TKIs is undefined, but the premature
terminations of the TATTON[28] [30] and CAURAL trials[29] [31]
due to the higher incidence of interstitial lung disease—like events with osimertinib and durvalumab provided
important information, leading to the consensus that
concurrent use should be avoided outside clinical trials.
Common practice to reduce pneumonitis risk is to defer
the TKI initiation for at least 1 month, preferably 3
months for less aggressive diseases, after the last use of immunotherapy.[7] [32] Extra caution is needed with the
third-generation TKI osimertinib compared to first- or
second-generation TKIs, especially in patients with
preexisting lung injuries.[32]
Limitations
Limitations of our study included small sample size,
variable follow-up, and assessment tools, leading to
inconsistent evaluations of efficacy and toxicities. The
unexpectedly low EGFR mutation rate (~30%) among
those tested makes it challenging to draw statistically
significant conclusions about the benefits for the
controversial EGFRm subgroup, despite an observed
improvement in PFS. Retrospective EGFR analysis
of the 37 untested cases could enhance understanding,
though further EGFR population enrichment may be
limited due to the expected low mutation rates based on
histology[33] (70.3% squamous, 8.1% lymphoepithelioma-like
carcinoma, 5.4% large cell, and 13.5% NSCLC of
no specific type). The imbalance and deviation in PD-L1
expression pattern, probably due to small sample size,
may also confound results. Collaborative multi-centre
analysis, adoption of universal EGFR testing for non-squamous
NSCLC, and increased accessibility of PD-L1
test in Hong Kong oncology centres could enhance the
statistical value of future similar studies by reducing the
untested population and increasing the overall sample size.
CONCLUSION
This study provides compelling evidence that
durvalumab consolidation therapy following CRT
improves PFS in unresectable stage III NSCLC, with
manageable adverse effects. Pneumonitis, occurring
mainly within the first 3 months, underscores the need
for close monitoring and timely management, especially
at the start of durvalumab. Lung V20Gy may predict
pneumonitis and should be kept as low as possible
after balancing a reasonable target coverage, but its
low specificity suggests it should be used alongside
other clinical factors for individual risk assessment and
planning.
As the treatment landscape for locally advanced NSCLC
is evolving, therapies effective in metastatic disease are
applied earlier in the treatment pathway. The recently
published LAURA study,[34] [35] which demonstrated a
highly encouraging PFS benefit from 5.6 months to
39.1 months with adjuvant osimertinib in EGFRm
patients, is probably just the start. With increasing
evidence, both PD-L1 and EGFR status are expected to
be critical in the near future to guide treatment selection. Further large-scale studies and uniform follow-up are
needed to validate the roles of different biomarkers in
tailoring treatments for patients with unresectable stage
III NSCLC, similar to the approach in stage IV disease.
REFERENCES
1. ClinicalTrials.gov. A Global Study to Assess the Effects of
MEDI4736 Following Concurrent Chemoradiation in Patients With
Stage III Unresectable Non-Small Cell Lung Cancer (PACIFIC).
Available from: https://clinicaltrials.gov/study/NCT02125461.
Accessed 21 May 2025.
2. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC
trial: durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. J Clin Oncol. 2022;40:1301-11. Crossref
3. ClinicalTrials.gov. First Real-world Data on Unresectable
Stage III NSCLC Patients Treated With Durvalumab After
Chemoradiotherapy. Available from: https://clinicaltrials.gov/study/NCT03798535?term=NCT03798535&rank=1. Accessed 21 May 2025.
4. Girard N, Bar J, Garrido P, Garassino MC, McDonald F,
Mornex F, et al. Treatment characteristics and real-world
progression-free survival in patients with unresectable stage III
NSCLC who received durvalumab after chemoradiotherapy:
findings from the PACIFIC-R study. J Thorac Oncol. 2023;18:181-93. Crossref
5. Hong Kong SAR Government. LCQ15: Treatments and support
for patients with cancers and rare diseases. 2020 Jun 24.
Available from: https://www.info.gov.hk/gia/general/202006/24/P2020062400288.htm. Accessed 23 Jun 2024.
6. Naidoo J, Antonia S, Wu YL, Cho BC, Thiyagarajah P, Mann H,
et al. Brief report: durvalumab after chemoradiotherapy in
unresectable stage III EGFR-mutant NSCLC: a post hoc subgroup
analysis from PACIFIC. J Thorac Oncol. 2023;18:657-63. Crossref
7. Aredo JV, Mambetsariev I, Hellyer JA, Amini A, Neal JW, Padda SK, et al. Durvalumab for stage III EGFR-mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol. 2021;16:1030-41. Crossref
8. Yang H, Zhu J, Xiao R, Liu Y, Yu F, Cai L, et al. EGFR mutation
status in non–small-cell lung cancer receiving PD-1/PD-L1
inhibitors and its correlation with PD-L1 expression: a meta-analysis.
Cancer Immunol Immunother. 2022;71:1001-16. Crossref
9. Detterbeck FC. The eighth edition TNM stage classification for lung cancer: what does it mean on main street? J Thorac Cardiovasc Surg. 2018;155:356-9. Crossref
10. United States Department of Health and Human Services.
Common Terminology Criteria for Adverse Events (CTCAE)
Version 5.0. 2017 Nov 27. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 4 Aug 2024.
11. Jung HA, Noh JM, Sun JM, Lee SH, Ahn JS, Ahn MJ, et al. Real-world data of durvalumab consolidation after chemoradiotherapy in stage III non–small-cell lung cancer. Lung Cancer. 2020;146:23-9. Crossref
12. Park CK, Oh HJ, Kim YC, Kim YH, Ahn SJ, Jeong WG, et al. Korean real-world data on patients with unresectable stage III NSCLC treated with durvalumab after chemoradiotherapy:
PACIFIC-KR. J Thorac Oncol. 2023;18:1042-54. Crossref
13. Preti BT, Sanatani MS, Breadner D, Lakkunarajah S, Scott C, Esmonde-White C, et al. Real-world analysis of durvalumab after chemoradiation in stage III non–small-cell lung cancer. Curr Oncol. 2023;30:7713-21. Crossref
14. Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non–small-cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620-30. Crossref
15. Tuminello S, Sikavi D, Veluswamy R, Gamarra C, Lieberman-Cribbin W, Flores R, et al. PD-L1 as a prognostic biomarker in surgically resectable non–small-cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2020;9:1343-60. Crossref
16. Vrankar M, Zwitter M, Kern I, Stanic K. PD-L1 expression can be regarded as prognostic factor for survival of non–small-cell lung cancer patients after chemoradiotherapy. Neoplasma. 2018;65:140-6. Crossref
17. Zhao Y, Shi F, Zhou Q, Li Y, Wu J, Wang R, et al. Prognostic significance of PD-L1 in advanced non–small-cell lung carcinoma. Medicine (Baltimore). 2020;99:e23172. Crossref
18. Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in
patients with non–small-cell lung cancer: a meta-analysis. Transl
Lung Cancer Res. 2015;4:203-8. Crossref
19. Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, Martínez-Pérez A, Rodrigo JP, García-Pedrero JM, et al. Chemo-immunotherapy: a new trend in cancer treatment. Cancers (Basel).
2023;15:2912. Crossref
20. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436-43. Crossref
21. Oshiro Y, Mizumoto M, Sekino Y, Maruo K, Ishida T, Sumiya T,
et al. Risk factor of pneumonitis on dose-volume relationship for
chemoradiotherapy with durvalumab: multi-institutional research
in Japan. Clin Transl Radiat Oncol. 2021;29:54-9. Crossref
22. Gao RW, Day CN, Yu NY, Bush A, Amundson AC, Prodduturvar P, et al. Dosimetric predictors of pneumonitis in locally advanced non–small-cell lung cancer patients treated with chemoradiation followed by durvalumab. Lung Cancer. 2022;170:58-64. Crossref
23. Beattie J, Rizvi H, Fuentes P, Luo J, Schoenfeld A, Lin IH, et al.
Success and failure of additional immune modulators in steroid-refractory/
resistant pneumonitis related to immune checkpoint
blockade. J Immunother Cancer. 2021;9:e001884. Crossref
24. Liu T, Li S, Ding S, Qiu J, Ren C, Chen J, et al. Comparison of
post-chemoradiotherapy pneumonitis between Asian and non-Asian patients with locally advanced non–small-cell lung cancer:
a systematic review and meta-analysis. EClinicalMedicine.
2023;64:102246. Crossref
25. Faehling M, Schulz C, Laack H, Wolff T, Rückert A, Reck M, et al.
PACIFIC subgroup analysis: pneumonitis in stage III, unresectable
NSCLC patients treated with durvalumab vs. placebo after CRT.
Pneumologie. 2019;73(S 01):s-0039-1678247. Crossref
26. Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE,
et al. Impact of intensity-modulated radiation therapy technique for
locally advanced non–small-cell lung cancer: a secondary analysis
of the NRG oncology RTOG 0617 randomized clinical trial. J Clin
Oncol. 2017;35:56-62. Crossref
27. Jung J, Kim HY, Kim DG, Park SY, Ko AR, Han JY, et al.
Sequential treatment with an immune checkpoint inhibitor followed
by a small-molecule targeted agent increases drug-induced
pneumonitis. Cancer Res Treat. 2021;53:77-86. Crossref
28. Ahn MJ, Cho BC, Ou X, Walding A, Dymond AW, Ren S, et al.
Osimertinib plus durvalumab in patients with EGFR-mutated,
advanced NSCLC: a phase 1b, open-label, multicenter trial. J
Thorac Oncol. 2022;17:718-23. Crossref
29. Yang JC, Shepherd FA, Kim DW, Lee GW, Lee JS, Chang GC, et al.
Osimertinib plus durvalumab versus osimertinib monotherapy in
EGFR T790M–positive NSCLC following previous EGFR TKI
therapy: CAURAL brief report. J Thorac Oncol. 2019;14:933-9. Crossref
30. ClinicalTrials.gov. AZD9291 in Combination With Ascending Doses of Novel Therapeutics. Available from: https://clinicaltrials.gov/study/NCT02143466. Accessed 21 May 2025.
31. ClinicalTrials.gov. Study of AZD9291 Plus MEDI4736 Versus
AZD9291 Monotherapy in NSCLC After Previous EGFR TKI
Therapy in T790M Mutation Positive Tumours (CAURAL).
Available from: https://clinicaltrials.gov/study/NCT02454933. Accessed 21 May 2025.
32. Kalra A, Rashdan S. The toxicity associated with combining
immune check point inhibitors with tyrosine kinase inhibitors
in patients with non–small-cell lung cancer. Front Oncol.
2023;13:1158417. Crossref
33. Joshi A, Zanwar S, Noronha V, Patil VM, Chougule A, Kumar R, et al. EGFR mutation in squamous cell carcinoma of the lung: does it carry the same connotation as in adenocarcinomas? Onco Targets Ther. 2017;10:1859-63. Crossref
34. Lu S, Kato T, Dong X, Ahn MJ, Quang LV, Soparattanapaisarn N, et al. Osimertinib after chemoradiotherapy in stage III EGFR-mutated NSCLC. N Engl J Med. 2024;391:585-97. Crossref
35. ClinicalTrials.gov. A Global Study to Assess the Effects of Osimertinib Following Chemoradiation in Patients With Stage III
Unresectable Non-small Cell Lung Cancer (LAURA). Available
from: https://clinicaltrials.gov/study/NCT03521154. Accessed 21 May 2025.