Efficacy of Prophylactic Embolisation of Renal Angiomyolipomas Using Semi-automatic Segmentation for Volume Measurement
ORIGINAL ARTICLE
Hong Kong J Radiol 2025;28:Epub 17 November 2025
Efficacy of Prophylactic Embolisation of Renal Angiomyolipomas Using Semi-automatic Segmentation for Volume Measurement
PL Lam1, JC Ng1, KH Lee1, KKF Fung2, DHY Cho1
1 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China
2 Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
Correspondence: Dr PL Lam, Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR,
China. Email: lpl404@ha.org.hk
Submitted: 28 August 2024; Accepted: 29 October 2024. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. PLL acquired the data. All authors analysed the data. PLL drafted the manuscript. All authors
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, KKFF was not involved in the peer review process. Other authors have disclosed no conflicts of
interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-022-4). The requirement for informed patient consent was waived by the Board due to the retrospective nature of the research.
Abstract
Introduction
We aimed to assess the efficacy of prophylactic embolisation of renal angiomyolipomas (AMLs) by
determining post-embolisation rupture risk, as well as changes in total volume and in angiomyogenic and fatty
components using semi-automatic segmentation.
Methods
This was a retrospective study of 22 adult patients with prophylactic embolisation of AML performed
between January 2009 and January 2024. Patients were followed up for any post-embolisation rupture. Pre- and
post-embolisation computed tomography (CT) data were assessed using the open-source software 3D Slicer for
semi-automatic segmentation. Volumetric changes of AMLs were compared using the Wilcoxon signed-rank test
for paired data and Mann-Whitney U test for unpaired data. Spearman’s rank correlation coefficient was used to
identify any associations between variables.
Results
There were 25 prophylactic embolisations performed on the 22 adult patients with AML (18 females
[81.8%]), with a median age of 60.0 years (interquartile range [IQR], 15.0). No procedure-related complications
were encountered. The median follow-up was 49.0 months (IQR, 56.0) with no post-embolisation rupture. Pre- and
post-treatment median tumour volumes were 67.5 cm3 (IQR, 116.1) and 35.7 cm3 (IQR, 82.1), respectively. There
was a significant reduction in total tumour volume (41.4%), including angiomyogenic (73.6%) and fatty components
(14.0%) [all p < 0.001]. Factors associated with greater tumour volume reduction included a higher proportion of
angiomyogenic and a lower proportion of fatty components (both p < 0.001).
Conclusion
Prophylactic embolisation of AML effectively reduced tumour volume, with more significant changes
in its angiomyogenic than fatty components. No post-embolisation rupture was documented with a median follow-up of over 4 years.
Key Words: Angiomyolipoma; Embolization, therapeutic; Hemorrhage; Kidney neoplasms; Tumor burden
中文摘要
半自動分割體積測量法評估預防性栓塞治療腎血管平滑肌脂肪瘤的療效
林栢麟、吳昆倫、李家灝、馮建勳、曹慶恩
引言
釐本研究旨在透過半自動分割技術,評估腎血管平滑肌脂肪瘤(AML)預防性栓塞的療效,具體方法包括確定栓塞術後破裂風險以及腫瘤總體積、血管肌源性成分和脂肪成分的變化。
方法
本研究為回顧性研究,納入2009年1月至2024年1月期間接受AML預防性栓塞的22位成年患者。所有患者均接受隨訪,觀察栓塞術後是否發生破裂。我們採用開源軟件3D Slicer對栓塞術前及術後的電腦斷層掃描圖像進行半自動分割,並採用Wilcoxon 符號排序檢定(配對資料)和Mann-Whitney U 檢定(非配對資料)比較AML的體積變化,以及採用Spearman秩相關系數分析各變數間的相關性。
結果
22位成年AML患者(18位女性[81.8%])接受了25次預防性栓塞治療,中位年齡為60.0歲(四分位數間距[IQR]為15.0)。沒有發生手術相關併發症。中位隨訪時間為49.0個月(IQR為56.0),沒有發生栓塞後破裂。治療前後腫瘤體積中位數分別為67.5 cm3(IQR為116.1)及35.7 cm3(IQR為82.1)。腫瘤總體積顯著縮小(41.4%),其中血管肌源性成分縮小73.6%,脂肪成分縮小14.0% [所有p < 0.001]。腫瘤體積顯著縮小的相關因素包括血管肌源性成分比例較高和脂肪成分比例較低(兩者 p < 0.001)。
結論
預防性栓塞治療AML可有效縮小腫瘤體積,且血管肌源性成分的變化比脂肪組成的變化更為顯著。中位隨訪時間超過4年,未記錄到栓塞後破裂病例。
INTRODUCTION
Renal angiomyolipoma (AML) is the most common
benign solid renal tumour.[1] The majority (approximately
80%) occur sporadically, while the rest (approximately
20%) are associated with phakomatoses, most
commonly tuberous sclerosis.[2] AML belongs to the
family of tumours with perivascular epithelioid cellular
differentiation.[3] It typically contains both angiomyogenic
and fatty components, with the latter readily identifiable
in computed tomography (CT) due to its hypoattenuating
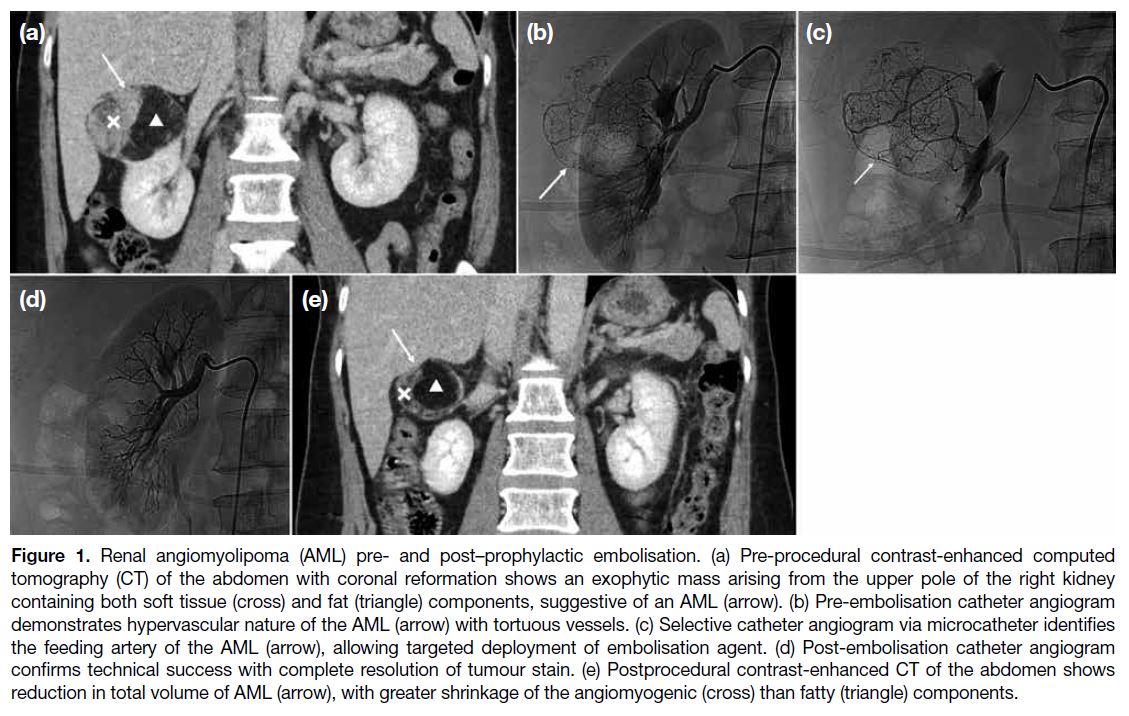
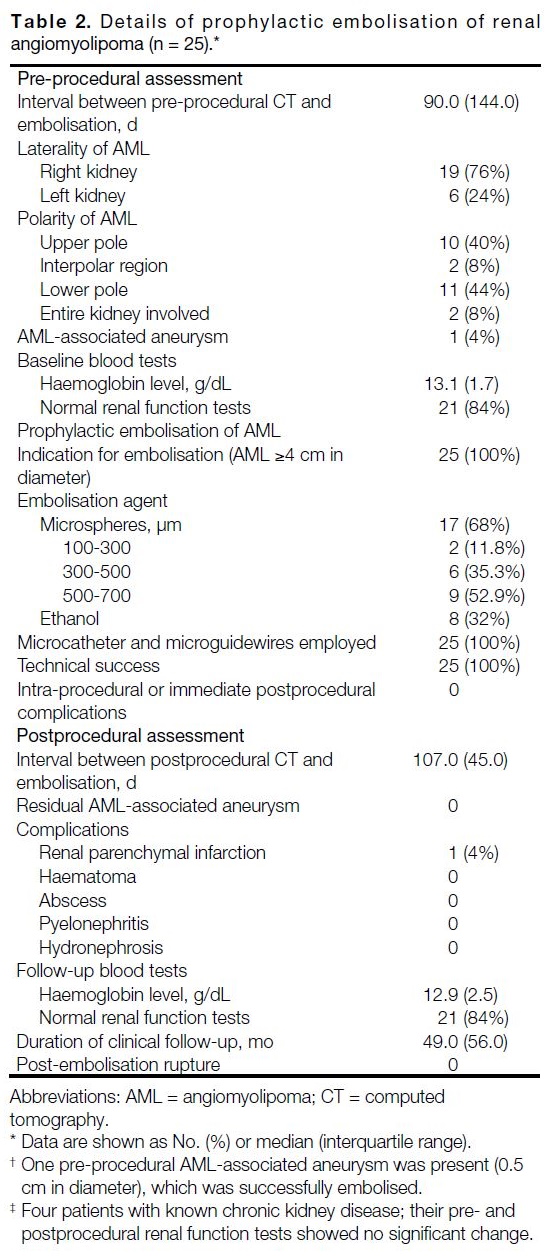
nature (< -10 Hounsfield unit [HU]) [Figure 1].[4] [5]
Figure 1. Renal angiomyolipoma (AML) pre- and post–prophylactic embolisation. (a) Pre-procedural contrast-enhanced computed
tomography (CT) of the abdomen with coronal reformation shows an exophytic mass arising from the upper pole of the right kidney
containing both soft tissue (cross) and fat (triangle) components, suggestive of an AML (arrow). (b) Pre-embolisation catheter angiogram
demonstrates hypervascular nature of the AML (arrow) with tortuous vessels. (c) Selective catheter angiogram via microcatheter identifies
the feeding artery of the AML (arrow), allowing targeted deployment of embolisation agent. (d) Post-embolisation catheter angiogram
confirms technical success with complete resolution of tumour stain. (e) Postprocedural contrast-enhanced CT of the abdomen shows
reduction in total volume of AML (arrow), with greater shrinkage of the angiomyogenic (cross) than fatty (triangle) components.
It is well recognised that AML carries a risk of rupture
with bleeding, especially for larger tumours, which can
lead to fatal consequences.[6] [7] Treatment options include
transcatheter arterial embolisation and radiofrequency
ablation, as well as partial or radical nephrectomy.[8]
Selective arterial embolisation can be performed in an
emergency setting for AML with active bleeding. It can
also be a prophylactic treatment to reduce tumour size
and its risk of haemorrhage (Figure 1).[9] [10] It has been
suggested that for AML of 4 cm or above in diameter, or
those with microaneurysms 0.5 cm or above in the feeding
artery, prophylactic selective arterial embolisation is indicated.[11] Different embolisation agents have been
reported in the literature, including microparticles, such
as microspheres, and liquid agents, such as ethanol. Past
studies have shown that prophylactic embolisation could
reduce the size of AML, thus reducing its haemorrhagic
risk.[9] [10] [12] In addition, the risk of haemorrhage is mainly
attributed to the angiomyogenic component of AML.[13] [14] [15]
Yet, there are limited studies accurately assessing how
tumour composition changes after treatment.
For AML, tumour size has been shown to be associated
with risk of spontaneous rupture, with larger ones
more likely to bleed.[6] [7] [11] This study therefore aimed
to assess the efficacy of prophylactic selective arterial
embolisation in determining the rupture risk post-embolisation
and reducing the volume of AML using
semi-automatic segmentation as a measurement tool.
Changes in its angiomyogenic and fatty components
were also evaluated.
METHODS
Patient Selection
This was a single-centre, single-arm retrospective
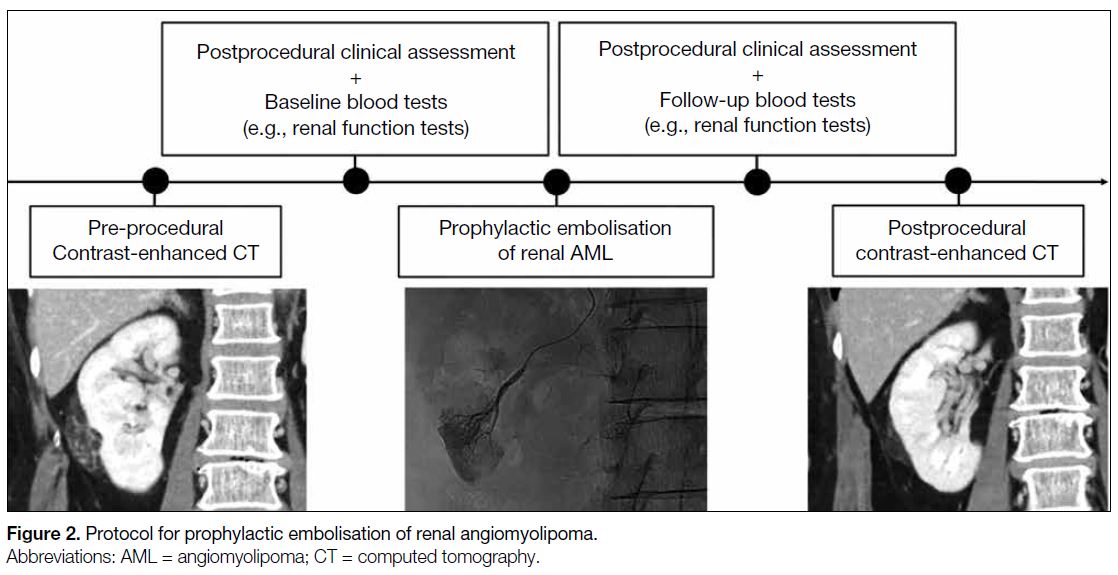
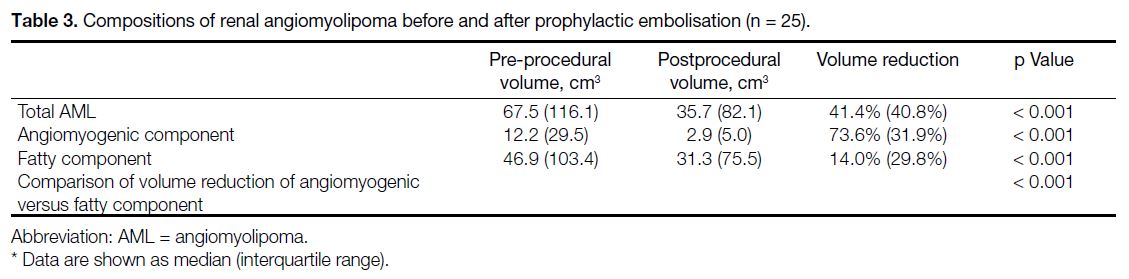
study. Consecutive adult patients (≥18 years old) who underwent prophylactic embolisation of AML (Figure 2) in a public acute general hospital between January
2009 and January 2024 were included. Exclusion criteria
included paediatric patients (<18 years old), patients
who underwent emergency embolisation of ruptured
AML, and patients without pre- or postprocedural CT.
Figure 2. Protocol for prophylactic embolisation of renal angiomyolipoma.
Data Collection
Clinical data of the included patients were retrieved from
the radiology information system of the hospital network.
They included demographics and medical history, such
as tuberous sclerosis status. Presenting symptoms and
postprocedural complaints were recorded. Pre- and post-intervention blood tests, such as haemoglobin level and
renal function tests, were documented.
Details of prophylactic embolisation of AML were
logged. They encompassed the type and amount of
embolisation agents deployed, as well as catheters
and guidewires used, which were chosen based on the
operators’ preference. Data on technical success, defined
as complete angiographic resolution of tumour stain
and microaneurysms, as well as contrast stasis of the
feeding artery, were documented. Intraoperative and
immediate postprocedural complications were recorded.
Subsequent clinical follow-up was reviewed for post-embolisation
tumour rupture.
Radiological Assessment
Pre- and post-embolisation plain and contrast-enhanced
CTs of the abdomen in DICOM (Digital Imaging and
Communications in Medicine) format were obtained
from the picture archiving and communication system
of the hospital network. The time interval between the
day of CT examination and interventional procedure
was logged. DICOM images were assessed using 3D
Slicer (macOS version 5.6.2; The Slicer Community),
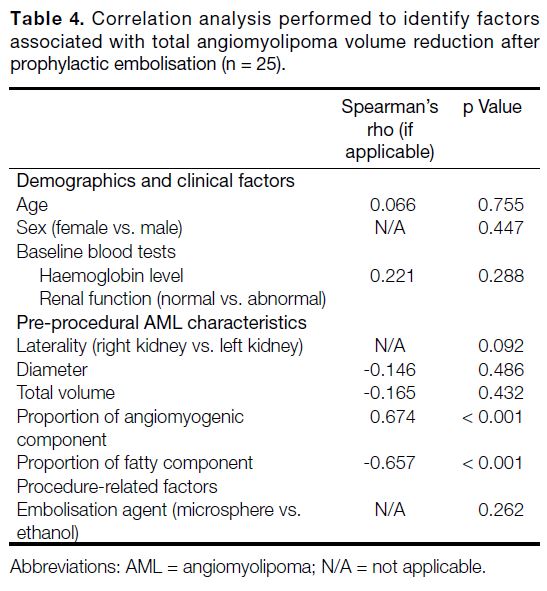
an open-source image computing platform.[16] Semi-automatic
segmentation of AMLs was performed in
the following sequence (Figure 3): (1) reformation of
contrast-enhanced CT images in axial, coronal and
sagittal planes; (2) manual contouring of tumour and
non-tumour regions on limited CT slices (<5); (3)
automatic segmentation of tumour and non-tumour
regions using the ‘grow from seeds’ algorithm; (4)
manual refinement of segmented regions using ‘paint’
and ‘erase’ algorithms; (5) automatic differentiation
between angiomyogenic and fatty components of AML
using a ‘threshold’ algorithm, with the threshold set
at ≥ -10 HU for the angiomyogenic component and
< -10 HU for the fatty component; (6) automatic volume
rendering of tumour and non-tumour regions; and (7)
automatic volumetric computation of the entire AML,
as well as its angiomyogenic and fatty components. In
addition, laterality and polarity of AML, as well as the
presence or absence of aneurysms, were documented.
In postprocedural CT, any complications, including
renal parenchymal infarction, haematoma, abscess,
pyelonephritis, or hydronephrosis, were recorded.
Figure 3. Semi-automatic segmentation of renal
angiomyolipoma (AML) using 3D Slicer. (a) Digital Imaging
and Communications in Medicine images of contrastenhanced
computed tomography (CT) of the abdomen
are reformatted in the axial (upper left), coronal (lower
left), and sagittal planes (lower right). An AML (arrows)
arises from the upper pole of the left kidney. (b) Contrast-enhanced
CT of the abdomen reformatted in sagittal
planes. Contours of AML (arrows) and other non-tumour
regions are drawn manually on several (<5) CT slices. (c)
Automatic segmentation of AML (arrows) is performed
in the axial (upper left), coronal (lower left), and sagittal
planes (lower right) using the ‘grow from seeds’ algorithm.
Further refinement of the segmented regions is possible
using the ‘paint’ and ‘erase’ algorithms. Automatic three-dimensional
rendering of (d) non-tumour regions and (e)
the AML are shown. Further volumetric analysis, such as
determining the angiomyogenic and fatty components of
the AML using -10 Hounsfield unit as the threshold, can
be performed.
Statistical Analysis
Statistical analysis was performed using SPSS (macOS
version 29.0; IBM Corp, Armonk [NY], United States).
The distribution of all numerical data was first tested for normality using the Shapiro-Wilk test. The Wilcoxon
signed-rank test was used to compare paired data,
such as pre- and postprocedural volumetric changes
in each AML. The Mann-Whitney U test was used for
comparison between unpaired data. Spearman’s rank
correlation coefficient was used to identify association
between variables. A p value of < 0.05 was considered
statistically significant.
This manuscript was prepared in accordance with the
STROBE (Strengthening the Reporting of Observational
Studies in Epidemiology) guidelines.
RESULTS
Patient Demographics and Clinical
Information
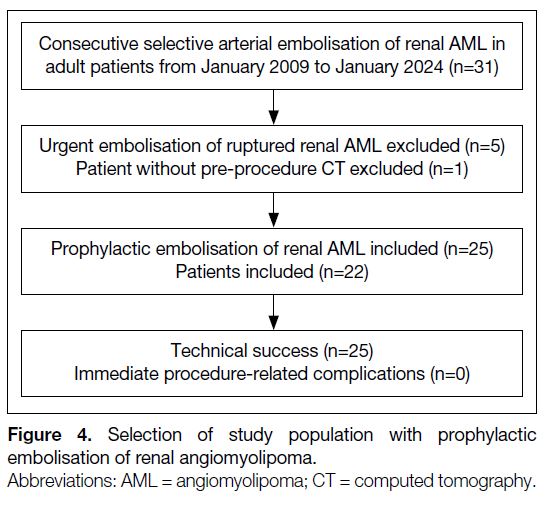
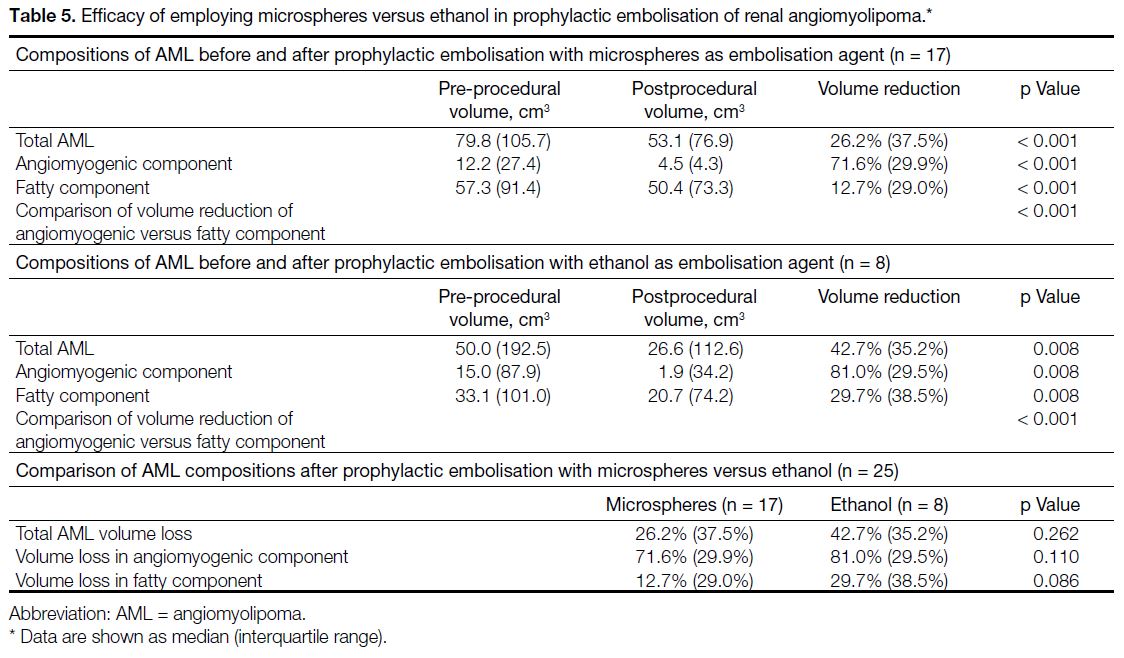
There were 31 prophylactic embolisations of AMLs
in adult patients performed from January 2009 to
January 2024. Five patients underwent emergent
embolisation of ruptured AMLs. One patient did not
have preoperative CT available for assessment. These
six patients were therefore excluded. A total of 25
prophylactic embolisations of AML in 22 patients were
finally included in the study, of which three patients had
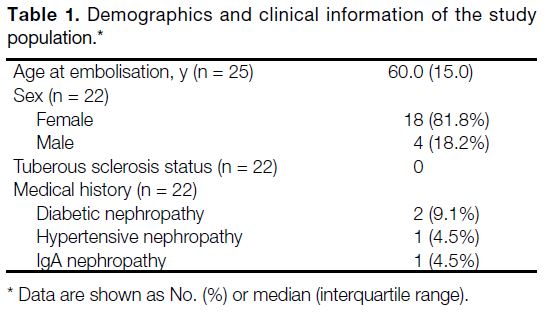
repeated embolisation (n = 3, 13.6%) [Figure 4]. The median age of the patients on the day of embolisation
was 60.0 years (interquartile range, 15.0). Most patients
were female (n = 18, 81.8%). There was no patient with
tuberous sclerosis. Four patients (18.2%) had known
chronic kidney disease due to diabetic nephropathy
(n = 2, 9.1%), hypertensive nephropathy (n = 1, 4.5%)
and IgA nephropathy (n = 1, 4.5%) [Table 1].
Figure 4. Selection of study population with prophylactic embolisation of renal angiomyolipoma.
Table 1. Demographics and clinical information of the study population.
Prophylactic Embolisation of Renal
Angiomyolipoma
All prophylactic embolisations of AMLs were performed
due to large tumour size (≥4 cm in diameter). In one
case (4.0%), there was a 0.5-cm aneurysm identified
in pre-procedural assessment, which was successfully
embolised. Microspheres (Embosphere Microsphere;
Merit Medical Systems, South Jordan [UT], United
States) were employed in two-thirds of all interventions
(n = 17, 68.0%). The sizes of the microparticles ranged
between 100-300 μm (n = 2, 11.8%), 300-500 μm
(n = 6, 35.3%) and 500-700 μm (n = 9, 52.9%). Ethanol
was used in the remaining one-third of the cases (n = 8,
32.0%). Ethanol was radio-opacified with ethiodised
oil in a ratio of 7:3 for embolisation of the other cases.
Selective arterial embolisation with microcatheters
and microguidewires was performed in every case.
All prophylactic embolisation achieved technical success. There were no intra-procedural or immediate
postprocedural complications encountered. The median
time intervals between pre- and postprocedural CT with
prophylactic embolisation were 90.0 days and 107.0 days,
respectively. Postprocedural CT showed a small (2.0 cm
in diameter) subsegmental renal infarction in one case
(4.0%). No other complications were seen. There were
no significant changes in haemoglobin level or renal
function tests before and after prophylactic embolisation.
Median clinical follow-up duration was over 4 years
(49.0 months), with a minimum of 6 months. There were
no post-embolisation rupture of AML (Table 2).
Table 2. Details of prophylactic embolisation of renal angiomyolipoma (n = 25).
Volumetric Analysis of Renal Angiomyolipoma
The median total volume of AMLs in pre- and
postprocedural CTs were 67.5 cm3 and 35.7 cm3,
respectively, showing significant interval shrinkage, with 41.4% total tumour volume reduction. Both
angiomyogenic and fatty components showed
significant interval reduction in size, attaining 73.6% and
14.0% volume loss, respectively. The angiomyogenic
component of the AMLs showed significantly greater
reduction in size compared to the fatty component
(Table 3).
Table 3. Compositions of renal angiomyolipoma before and after prophylactic embolisation (n = 25).
Correlation analysis revealed AMLs with a greater
proportion of angiomyogenic component and smaller
proportion of fatty component in pre-procedural CT
were associated with greater tumour volume reduction
after prophylactic embolisation. No other clinical or
procedural factors associated with total tumour shrinkage
were identified (Table 4).
Table 4. Correlation analysis performed to identify factors associated with total angiomyolipoma volume reduction after prophylactic embolisation (n = 25).
Prophylactic embolisation of AML with either
microspheres or ethanol achieved significant reduction
in total tumour size, with 26.2% (p < 0.001) and 42.7%
(p = 0.008) volume loss, respectively. Using either
embolisation agent, there were significant reduction
in volume of both angiomyogenic (microspheres:
71.6%, p < 0.001; ethanol: 81.0%, p = 0.008) and fatty
components (microspheres: 12.7%, p < 0.001; ethanol:
29.7%, p = 0.008), with the angiomyogenic component showing significantly greater volume loss than the fatty
component using either embolisation agent (both p < 0.001). Comparing microspheres and ethanol, there were
no statistically significant differences in their efficacy of
reduction of the total tumour volume, angiomyogenic or
fatty components of AML (Table 5).
Table 5. Efficacy of employing microspheres versus ethanol in prophylactic embolisation of renal angiomyolipoma.
DISCUSSION
Transcatheter embolisation of AML is recognised as
a safe intervention.[9] [10] Compared to more invasive
treatment options such as partial or radical nephrectomy,
transcatheter selective arterial embolisation typically
only requires local anaesthesia, has lower risks of
bleeding and infection, and allows shorter admission
times. Some authors therefore suggest transcatheter
embolisation as the first-line treatment option.[8] In our
study, no intra-procedural or immediate complications
were encountered. However, in one patient, a small
subsegmental renal infarction was seen in postprocedural
CT. This highlights the importance of follow-up imaging,
which encompasses assessment of treatment efficacy, as
well as identification of complications.
There was significant change in tumour volume after
prophylactic embolisation of AML, achieving over 40%
reduction in median total volume amongst our study
population. With decreased tumour volume, the risk of
spontaneous haemorrhage would be lowered.[6] [11] It was
reassuring that none of the included patients encountered
post-embolisation tumour rupture in clinical follow-up
with a median duration of over 4 years. These findings
demonstrate that prophylactic embolisation of AML is
a safe and effective means to reduce haemorrhagic risk,
concurrent with previous studies.[9] [10]
Various materials for prophylactic embolisation of
the kidney have been suggested in the literature,
with microspheres and ethanol being two of the
most commonly adopted agents. In this study, both microspheres and ethanol effectively reduced the size
of AMLs by over 25%, without a statistically significant
difference between the two agents. To the best of our
knowledge, there is no large-scale study establishing
whether microspheres or ethanol is the superior
prophylactic embolisation agent for AML.[9] [10] [12] In our centre, this choice depended on the operators’ preference.
It has been proposed that the effectiveness of
prophylactic embolisation in achieving volume reduction
depends on the composition of the AML, which has
variable angiomyogenic and fatty components. The
angiomyogenic component usually demonstrates
greater response to embolisation due to its vascular
nature, whereas the fatty component is hypovascular
and more treatment-resistant.[9] [17] A study by Han et al[17]
showed near-complete resolution of the angiomyogenic
component after prophylactic embolisation, but the
fatty component only partially shrank. In their study,
the proportion of angiomyogenic and fatty components
were evaluated on a transverse image at the middle
of the tumour. However, this might not reflect the
actual composition of the entire AML. In our study,
semi-automatic segmentation was performed, and the
angiomyogenic and fatty components were differentiated
using -10 HU as the threshold. This allowed a more accurate volumetric assessment of AML. Similar to
prior studies, there was significantly greater reduction in
the angiomyogenic component than the fatty component
after embolisation.
AMLs with a greater proportion of angiomyogenic
component and smaller proportion of fatty component
are associated with greater total volume reduction
after embolisation. For AML with high fatty content,
patients and clinicians may be concerned that about the
smaller postprocedural volume reduction. However,
the angiomyogenic component of AML, which is the
main culprit in haemorrhage, has shown good response
to embolisation.[9] [17] In our study, the angiomyogenic
component achieved over 70% volume reduction, which
could be reassuring to both patients and clinicians.
Limitations
First, none of the patients in our study had tuberous
sclerosis. Treatment efficacy for sporadic and tuberous
sclerosis–associated AML may differ and have not
been explored. Second, there was a lack of a control
group to compare rupture risk in patients who received
prophylactic embolisation versus those who did not. A
double-arm study could better assess treatment effect.
Third, the sample size was limited. This may be partly attributed to the relatively low prevalence of AML, which
is below 0.5% in the population.[18] A multi-centre study
with larger sample sizes is a potential future direction.
CONCLUSION
Prophylactic embolisation of AML effectively reduced
tumour volume, with more significant changes in the
angiomyogenic component compared to the fatty
component. No rupture or haemorrhage was documented
post-embolisation with a median follow-up of over 4
years.
REFERENCES
1. Prasad SR, Surabhi VR, Menias CO, Raut AA, Chintapalli KN.
Benign renal neoplasms in adults: cross-sectional imaging findings.
AJR Am J Roentgenol. 2008;190:158-64. Crossref
2. Steiner MS, Goldman SM, Fishman EK, Marshall FF. The natural history of renal angiomyolipoma. J Urol. 1993;150:1782-6. Crossref
3. Chan TY. World Health Organization classification of tumours: pathology & genetics of tumours of the urinary system and male
genital organs. Urology. 2005;65:214-5. Crossref
4. Halpenny D, Snow A, McNeill G, Torreggiani WC. The radiological diagnosis and treatment of renal angiomyolipoma—current status.
Clin Radiol. 2010;65:99-108. Crossref
5. Park BK. Renal angiomyolipoma: radiologic classification and
imaging features according to the amount of fat. AJR Am J
Roentgenol. 2017;209:826-35. Crossref
6. Ruud Bosch JL, Vekeman F, Duh MS, Neary M, Magestro M, Fortier J, et al. Factors associated with the number and size of renal
angiomyolipomas in sporadic angiomyolipoma (sAML): a study
of adult patients with sAML managed in a Dutch tertiary referral
center. Int Urol Nephrol. 2018;50:459-67. Crossref
7. Wang C, Li X, Peng L, Gou X, Fan J. An update on recent developments in rupture of renal angiomyolipoma. Medicine
(Baltimore). 2018;97:e0497. Crossref
8. Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, et al. Update on the diagnosis and management of
renal angiomyolipoma. J Urol. 2016;195:834-46. Crossref
9. Kothary N, Soulen MC, Clark TW, Wein AJ, Shlansky-Goldberg RD, Crino PB, et al. Renal angiomyolipoma: long-term results after
arterial embolization. J Vasc Interv Radiol. 2005;16:45-50. Crossref
10. Lenton J, Kessel D, Watkinson AF. Embolization of renal angiomyolipoma: immediate complications and long-term
outcomes. Clin Radiol. 2008;63:864-70. Crossref
11. Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M,
Takeda K. Renal angiomyolipoma: relationships between tumor
size, aneurysm formation, and rupture. Radiology. 2002;225:78-82. Crossref
12. Chatziioannou A, Gargas D, Malagari K, Kornezos I, Ioannidis I,
Primetis E, et al. Transcatheter arterial embolization as therapy of
renal angiomyolipomas: the evolution in 15 years of experience.
Eur J Radiol. 2012;81:2308-12. Crossref
13. Combes A, McQueen S, Palma CA, Benz D, Leslie S, Sved P, et al.
Is size all that matters? New predictors of complications and
bleeding in renal angiomyolipoma. Res Rep Urol. 2023;15:113-21. Crossref
14. Xu XF, Hu XH, Zuo QM, Zhang J, Xu HY, Zhang Y. A scoring
system based on clinical features for the prediction of sporadic renal
angiomyolipoma rupture and hemorrhage. Medicine (Baltimore).
2020;99:e20167. Crossref
15. Rimon U, Duvdevani M, Garniek A, Golan G, Bensaid P, Ramon J, et al. Large renal angiomyolipomas: digital subtraction
angiographic grading and presentation with bleeding. Clin Radiol.
2006;61:520-6. Crossref
16. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC,
Pujol S, et al. 3D Slicer as an image computing platform for
the Quantitative Imaging Network. Magn Reson Imaging.
2012;30:1323-41. Crossref
17. Han YM, Kim JK, Roh BS, Song HY, Lee JM, Lee YH, et al. Renal
angiomyolipoma: selective arterial embolization—effectiveness
and changes in angiomyogenic components in long-term follow-up.
Radiology. 1997;204:65-70. Crossref
18. Fittschen A, Wendlik I, Oeztuerk S, Kratzer W, Akinli AS, Haenle MM, et al. Prevalence of sporadic renal angiomyolipoma:
a retrospective analysis of 61,389 in- and out-patients. Abdom
Imaging. 2014;39:1009-13. Crossref