Multimodality Imaging and Interventional Radiological Management of Neurologic Complications of Infective Endocarditis
PICTORIAL ESSAY
Hong Kong J Radiol 2025;28:Epub 9 December 2025
Multimodality Imaging and Interventional Radiological
Management of Neurologic Complications of Infective Endocarditis
EH Chan, HM Kwok, NY Pan, LF Cheng, JKF Ma
Department of Diagnostic and Interventional Radiology, Kowloon West Cluster, Hong Kong SAR, China
Correspondence: Dr EH Chan, Department of Diagnostic and Interventional Radiology, Kowloon West Cluster, Hong Kong SAR,
China. Email: eh278@ha.org.hk
Submitted: 2 September 2024; Accepted: 1 November 2024. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study, acquired and analysed the data. EHC drafted the manuscript. All authors critically revised the
manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-012-4). The requirement for patient consent was waived by the Board due to retrospective nature of the study.
INTRODUCTION
Infective endocarditis (IE) affects 1.7 to 6.2 individuals
per 100,000 population per year and remains a
life-threatening condition.[1] Staphylococci are the
most frequent causative organisms.[1] [2] Neurological
complications are the most common and severe
extracardiac complications of IE[3] and have been reported
as the presenting symptom in up to 47% of cases.[4] These
complications are caused by cerebral septic embolisation
of endocardial vegetations. Patients with neurological
complications have significantly higher mortality
compared to those without (24% vs. 10%; p < 0.03).[3]
Neuroimaging leads to the identification of valvular
surgery indications in about 22% of patients with
symptoms of neurological complications of IE, and in
19% of asymptomatic IE patients.[2] Up to 82% of patients
have cerebral lesions on magnetic resonance imaging
(MRI) performed within 7 days after admission.[1]
MRI findings influence diagnostic classification and
other clinical decisions in 28% of patients, including
modification of medical or surgical treatment plans.[5]
According to the 2015 European Society of Cardiology guidelines,[6] the presence of cerebral emboli in patients
with left-sided valvular vegetations greater than 10
mm is an indication for urgent valve surgery to prevent
further embolisms. Familiarity with the neurological
imaging findings is essential for early diagnosis of this
complication of IE, allowing a window for early and
specific treatment, thereby reducing mortality. However,
the wide spectrum of presentations on neuroimaging
poses diagnostic challenges to radiologists, especially
when cerebral septic embolism is the first presentation.
This pictorial essay aims to review the spectrum of
presentations and the use of multimodality imaging to
increase awareness of the classic diagnostic imaging
findings of cerebral septic emboli secondary to IE, and to
highlight the role of interventional radiology in clinical
management.
Diagnosis
The diagnosis of IE is made according to the Modified
Duke criteria,[1] which include the presence of major
arterial emboli, mycotic aneurysm, and intracranial
haemorrhage as part of its minor criteria.
Computed tomography (CT) is the first-line imaging
study in patients with neurological symptoms as it is
readily available. MRI, including susceptibility-weighted
imaging (SWI) and diffusion-weighted imaging (DWI),
is required to detect more subtle findings such as cerebral
microbleeds and early infarcts. Further investigations
with computed tomographic angiography (CTA) or
magnetic resonance angiography (MRA) are useful for
detecting mycotic aneurysms, while digital subtraction
angiography (DSA) remains the gold standard and
should be performed in clinically suspicious cases with
negative CTA or MRA.[7] There is growing support for
performing screening MRI in patients with suspected
or confirmed IE, given the frequency of asymptomatic
findings and its usefulness in decision-making.
However, its cost-effectiveness and impact on mortality
reduction remain to be seen.[8]
Imaging Spectrum
Neurological complications of IE may present as cerebral
infarcts, micro- or macro-haemorrhage, abscess, and
meningitis. The pooled frequency of individual findings
on MRI is as follows: acute ischaemic lesions (61.9%),
cerebral microbleeds (52.9%), macro-haemorrhages
(24.7%), abscess or meningitis (9.5%), and intracranial
mycotic aneurysm (6.2%).[8] Accurate identification of these lesions allows early diagnosis of IE
complications and individualised management strategies.
Ischaemic Stroke
Ischaemic stroke is the most common neurological
manifestation of IE. It can result from embolisation
of endocardial vegetations, leading to occlusion of
intracerebral arteries.[3] The incidence of cerebral
ischaemia is correlated with left-sided endocarditis
(especially involving the anterior mitral valve leaflet),
larger endocardial vegetation size (>10 mm), mobile
vegetations, and Staphylococcus aureus infection.[3]
Disseminated ischaemic lesions may result from multiple
emboli occurring over a short period or fragmentation of
an embolus in the heart or aorta. The presence of multiple
cortical and subcortical cerebral infarcts of varying
ages within different vascular territories (especially
watershed areas) or bihemispheric involvement suggests
the diagnosis of septic emboli[4] (Figure 1). Large emboli
tend to cause cortical infarction in the middle cerebral
artery (MCA) territory, while smaller emboli often lodge
distally in terminal cortical branches of the anterior
cerebral artery and MCA, resulting in small peripheral
infarcts at the grey-white matter junction.[9] It is worth
noting that isolated brainstem strokes are rarely caused
by cardioembolism.
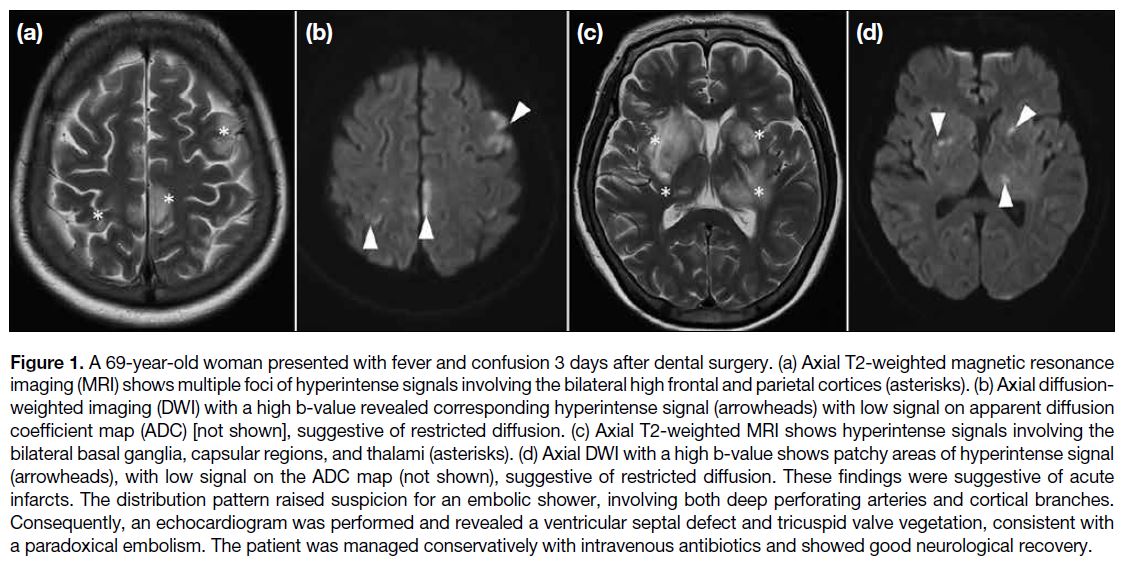
Figure 1. A 69-year-old woman presented with fever and confusion 3 days after dental surgery. (a) Axial T2-weighted magnetic resonance
imaging (MRI) shows multiple foci of hyperintense signals involving the bilateral high frontal and parietal cortices (asterisks). (b) Axial diffusion-weighted
imaging (DWI) with a high b-value revealed corresponding hyperintense signal (arrowheads) with low signal on apparent diffusion
coefficient map (ADC) [not shown], suggestive of restricted diffusion. (c) Axial T2-weighted MRI shows hyperintense signals involving the
bilateral basal ganglia, capsular regions, and thalami (asterisks). (d) Axial DWI with a high b-value shows patchy areas of hyperintense signal
(arrowheads), with low signal on the ADC map (not shown), suggestive of restricted diffusion. These findings were suggestive of acute
infarcts. The distribution pattern raised suspicion for an embolic shower, involving both deep perforating arteries and cortical branches.
Consequently, an echocardiogram was performed and revealed a ventricular septal defect and tricuspid valve vegetation, consistent with
a paradoxical embolism. The patient was managed conservatively with intravenous antibiotics and showed good neurological recovery.
DWI is useful for assessing the temporal relationship of
ischaemic lesions. Acute infarcts appear as hyperintense
on DWI and hypointense on apparent diffusion coefficient mapping. Over time, the apparent diffusion coefficient
signal increases and pseudonormalises in about 1 week,
signal increases and pseudonormalises in about 1 week,
while the DWI signal decreases and pseudonormalises in
about 2 weeks.[10]
Cerebral Abscesses and Meningitis
Cerebral abscesses and meningitis are uncommon
neurological manifestations of IE, occurring in up to
9.5% of patients.[8]
Typically, multiple abscesses appear in the MCA
territory at the grey-white matter junction, often with
vasogenic oedema and associated mass effect or haemorrhage.[3] On CT, cerebral abscesses are usually
hypodense with ring enhancement, but MRI is more
sensitive. Classic MRI features include lesions that are
hypointense on T1-weighted images and hyperintense
on T2-weighted images, with ring enhancement and
central restricted diffusion (Figure 2). A dual rim sign,
two concentric rims surrounding the abscess cavity,
where the outer rim is hypointense and the inner is
relatively hyperintense, may be visible on SWI or T2-weighted imaging. Cerebral abscesses may also arise
near mycotic aneurysms (Figures 3 and 4). The presence
of leptomeningeal enhancement on MRI or CT can
suggest concomitant meningitis.
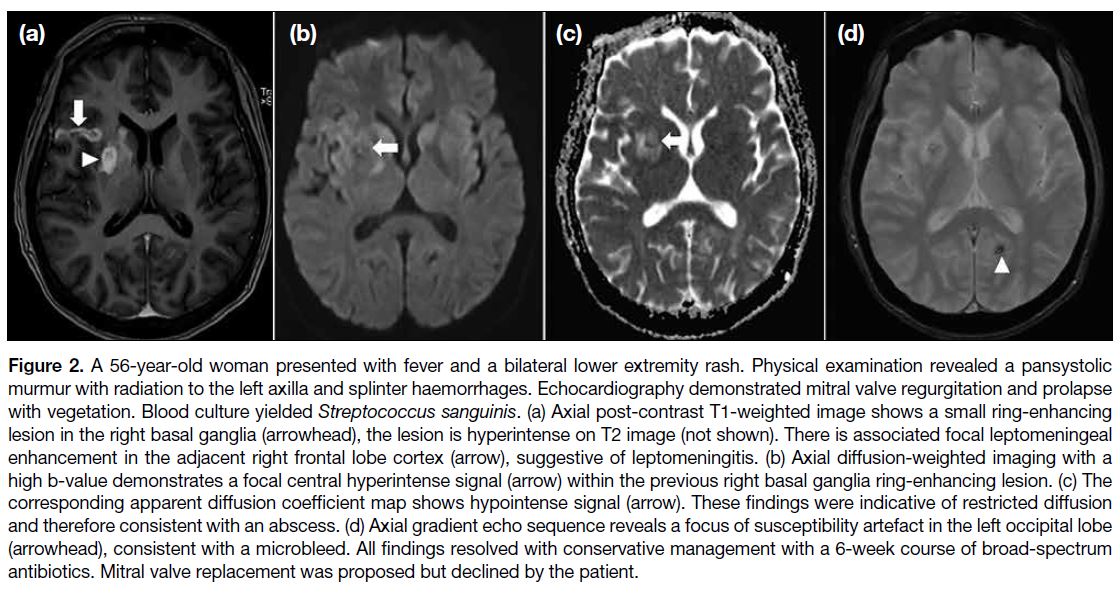
Figure 2. A 56-year-old woman presented with fever and a bilateral lower extremity rash. Physical examination revealed a pansystolic
murmur with radiation to the left axilla and splinter haemorrhages. Echocardiography demonstrated mitral valve regurgitation and prolapse
with vegetation. Blood culture yielded Streptococcus sanguinis. (a) Axial post-contrast T1-weighted image shows a small ring-enhancing
lesion in the right basal ganglia (arrowhead), the lesion is hyperintense on T2 image (not shown). There is associated focal leptomeningeal
enhancement in the adjacent right frontal lobe cortex (arrow), suggestive of leptomeningitis. (b) Axial diffusion-weighted imaging with a
high b-value demonstrates a focal central hyperintense signal (arrow) within the previous right basal ganglia ring-enhancing lesion. (c) The
corresponding apparent diffusion coefficient map shows hypointense signal (arrow). These findings were indicative of restricted diffusion
and therefore consistent with an abscess. (d) Axial gradient echo sequence reveals a focus of susceptibility artefact in the left occipital lobe
(arrowhead), consistent with a microbleed. All findings resolved with conservative management with a 6-week course of broad-spectrum
antibiotics. Mitral valve replacement was proposed but declined by the patient.
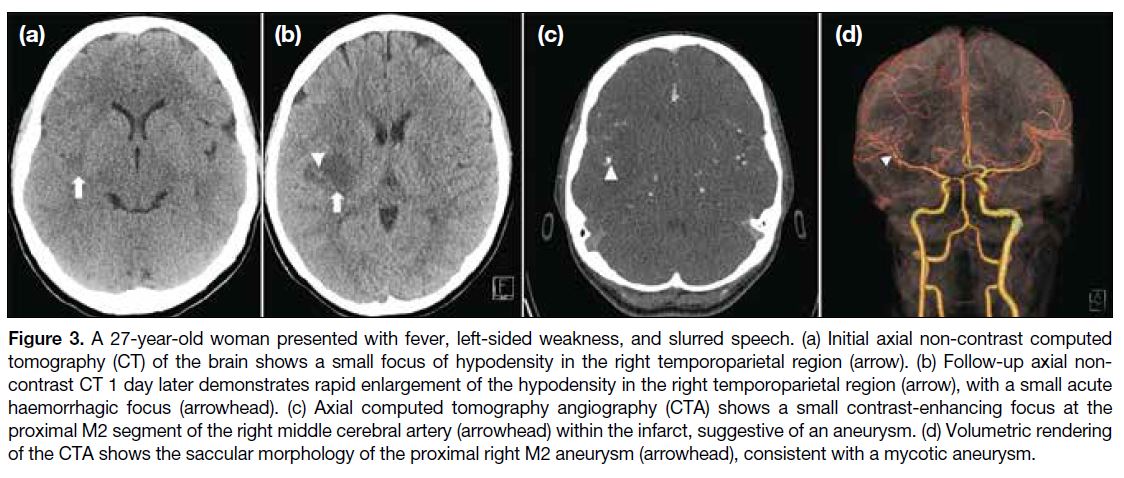
Figure 3. A 27-year-old woman presented with fever, left-sided weakness, and slurred speech. (a) Initial axial non-contrast computed
tomography (CT) of the brain shows a small focus of hypodensity in the right temporoparietal region (arrow). (b) Follow-up axial non-contrast
CT 1 day later demonstrates rapid enlargement of the hypodensity in the right temporoparietal region (arrow), with a small acute
haemorrhagic focus (arrowhead). (c) Axial computed tomography angiography (CTA) shows a small contrast-enhancing focus at the
proximal M2 segment of the right middle cerebral artery (arrowhead) within the infarct, suggestive of an aneurysm. (d) Volumetric rendering
of the CTA shows the saccular morphology of the proximal right M2 aneurysm (arrowhead), consistent with a mycotic aneurysm.
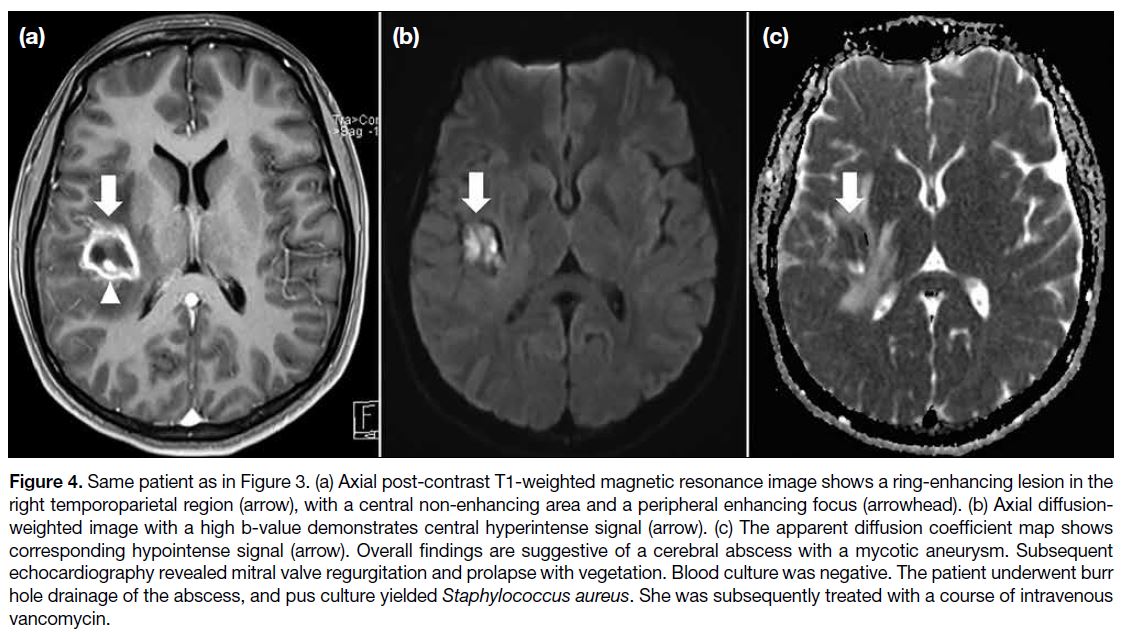
Figure 4. Same patient as in Figure 3. (a) Axial post-contrast T1-weighted magnetic resonance image shows a ring-enhancing lesion in the
right temporoparietal region (arrow), with a central non-enhancing area and a peripheral enhancing focus (arrowhead). (b) Axial diffusion-weighted
image with a high b-value demonstrates central hyperintense signal (arrow). (c) The apparent diffusion coefficient map shows
corresponding hypointense signal (arrow). Overall findings are suggestive of a cerebral abscess with a mycotic aneurysm. Subsequent
echocardiography revealed mitral valve regurgitation and prolapse with vegetation. Blood culture was negative. The patient underwent burr
hole drainage of the abscess, and pus culture yielded Staphylococcus aureus. She was subsequently treated with a course of intravenous
vancomycin.
Cerebral Haemorrhages
Macrohaemorrhage usually results from haemorrhagic
transformation of ischaemic stroke, progression of
microhaemorrhages, or rupture of mycotic aneurysms.
Haemorrhagic transformation occurs more frequently in
embolic strokes (51%-71%) than in non-embolic strokes
(2%-21%)[11] and may present as petechial haemorrhage
or large parenchymal haematomas.[9] Cerebral ischaemic
lesions of varying ages across multiple vascular
territories and different haemorrhagic patterns would
raise suspicion for cardiac emboli. In the context of
underlying IE, cerebral septic embolism is a likely
diagnosis (Figure 5).
Septic emboli damage the endothelium and disrupt the
blood-brain barrier, resulting in inflammatory vasculitis
and small vessel rupture, often leading to cerebral
microbleeds or even intracerebral haemorrhage. One
study found that cerebral microbleeds in 57% of patients
with IE.[4] These microbleeds appear as hypointense foci
on T2* imaging or SWI MRI often in the cortex, and less
frequently in subcortical white matter, basal ganglia, or
posterior fossa.[4]
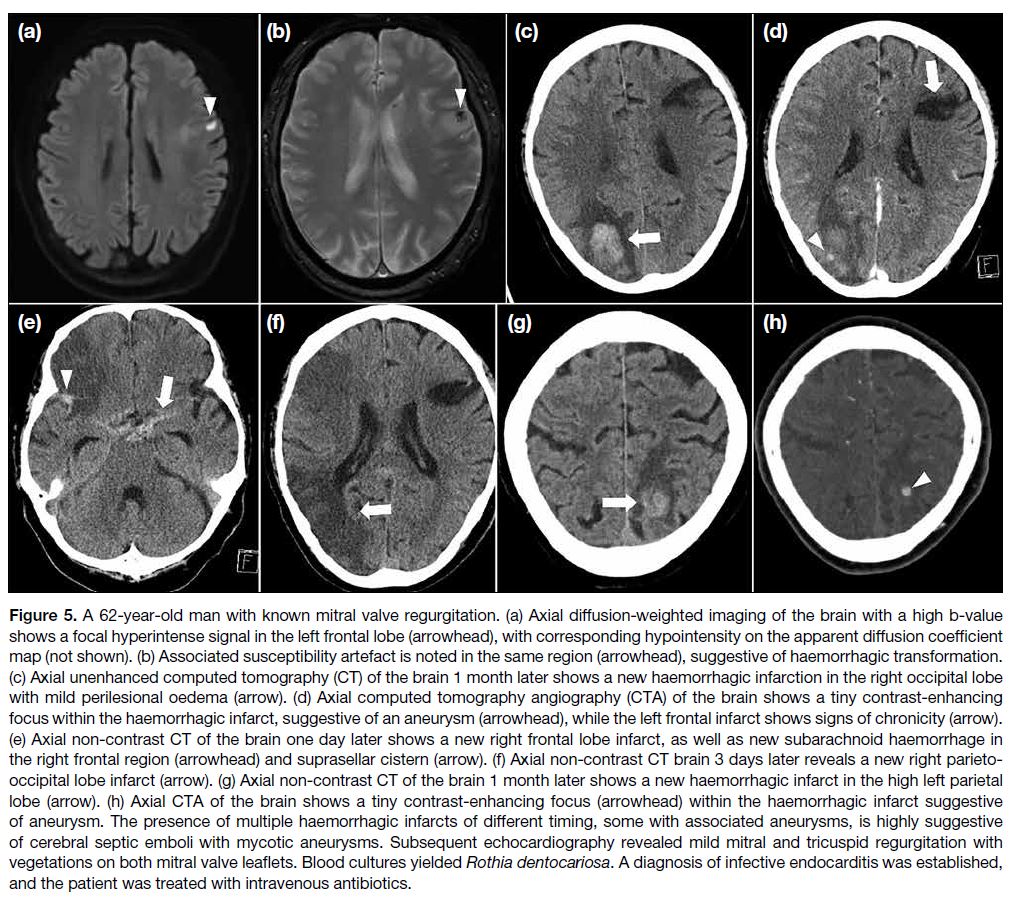
Figure 5. A 62-year-old man with known mitral valve regurgitation. (a) Axial diffusion-weighted imaging of the brain with a high b-value
shows a focal hyperintense signal in the left frontal lobe (arrowhead), with corresponding hypointensity on the apparent diffusion coefficient
map (not shown). (b) Associated susceptibility artefact is noted in the same region (arrowhead), suggestive of haemorrhagic transformation.
(c) Axial unenhanced computed tomography (CT) of the brain 1 month later shows a new haemorrhagic infarction in the right occipital lobe
with mild perilesional oedema (arrow). (d) Axial computed tomography angiography (CTA) of the brain shows a tiny contrast-enhancing
focus within the haemorrhagic infarct, suggestive of an aneurysm (arrowhead), while the left frontal infarct shows signs of chronicity (arrow).
(e) Axial non-contrast CT of the brain one day later shows a new right frontal lobe infarct, as well as new subarachnoid haemorrhage in
the right frontal region (arrowhead) and suprasellar cistern (arrow). (f) Axial non-contrast CT brain 3 days later reveals a new right parieto-occipital
lobe infarct (arrow). (g) Axial non-contrast CT of the brain 1 month later shows a new haemorrhagic infarct in the high left parietal
lobe (arrow). (h) Axial CTA of the brain shows a tiny contrast-enhancing focus (arrowhead) within the haemorrhagic infarct suggestive
of aneurysm. The presence of multiple haemorrhagic infarcts of different timing, some with associated aneurysms, is highly suggestive
of cerebral septic emboli with mycotic aneurysms. Subsequent echocardiography revealed mild mitral and tricuspid regurgitation with
vegetations on both mitral valve leaflets. Blood cultures yielded Rothia dentocariosa. A diagnosis of infective endocarditis was established,
and the patient was treated with intravenous antibiotics.
Mycotic Aneurysms
Cerebral septic emboli can trigger inflammation and
weakening of vessel walls, forming mycotic aneurysms.[7] These aneurysms are found in about 6.2% of patients with
IE and may shrink, enlarge, or develop de novo within
1 week to 3 months of starting antibiotics.[12] Mycotic
aneurysms have a 2% to 10% risk of rupture regardless
of their size and are associated with a high mortality
rate of 80%.[7] About 22% of IE patients presenting with
intracerebral haemorrhage have mycotic aneurysms
which should be promptly identified.[13] CTA or MRA
should be performed to confirm the diagnosis (Figures 5 and 6), followed by DSA for clear delineation of the
number, size and location of the mycotic aneurysms and
surgical or endovascular planning.
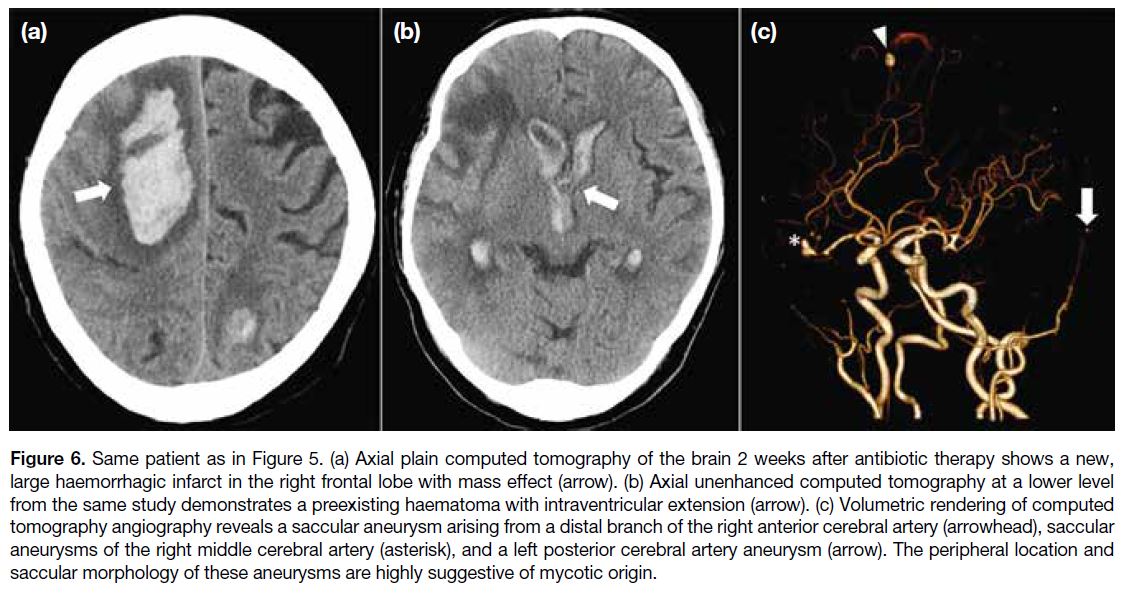
Figure 6. Same patient as in Figure 5. (a) Axial plain computed tomography of the brain 2 weeks after antibiotic therapy shows a new,
large haemorrhagic infarct in the right frontal lobe with mass effect (arrow). (b) Axial unenhanced computed tomography at a lower level
from the same study demonstrates a preexisting haematoma with intraventricular extension (arrow). (c) Volumetric rendering of computed
tomography angiography reveals a saccular aneurysm arising from a distal branch of the right anterior cerebral artery (arrowhead), saccular
aneurysms of the right middle cerebral artery (asterisk), and a left posterior cerebral artery aneurysm (arrow). The peripheral location and
saccular morphology of these aneurysms are highly suggestive of mycotic origin.
CTA and MRA have low sensitivity for small (<5 mm)
or distal mycotic aneurysms.[1] Aneurysms near the skull
base may be overlooked on CTA, while those in low-flow
areas may be missed on time-of-flight MRA.[14] In
cases with clinical suspicion of mycotic aneurysm but
negative CTA or MRA, DSA should be performed.[1]
Features favouring mycotic aneurysms include
multiplicity, saccular shape, distal location (such as
MCA segments 2 to 4 or posterior cerebral artery), size
or morphological changes on consecutive angiograms,
presence of other intra- or extra-cranial mycotic
aneurysms, adjacent arterial occlusion or stenosis, and
cerebral infarction at the aneurysm site[13] (Figure 6).
Management
Neurological complications from IE are life-threatening
and require multidisciplinary management, involving
neurosurgeons, radiologists, cardiologists, and
microbiologists. Empirical intravenous antibiotic therapy
is promptly administered and later tailored according
to culture sensitivity results. Valvular replacement
combined with antibiotics yield better outcomes than
antibiotics alone in left-sided endocarditis.[15]
Radiologists play a key role in both diagnosis and guiding
treatment by accurately reporting the type and severity
of each lesion. Surgical drainage can be considered in
cases of cerebral abscesses with significant mass effect.
Antiplatelet drugs and anticoagulants are contraindicated in both ischaemic stroke and macrohaemorrhage caused
by septic embolism due to the high risk of bleeding.[3]
Cardiac surgery should be postponed for at least 4 weeks
after a clinically significant intracranial haemorrhage
or large ischaemic infarct.[3] Mycotic aneurysms should
be excluded before open heart surgery for valvular
replacement requiring anticoagulation to reduce bleeding
risk.[15]
Interventional radiologists play an evolving role of
in treating mycotic aneurysms in collaboration with
neurosurgeons. Techniques include preoperative CTA or
MRA with volumetric rendering, road-map technique for
neuro-navigation, and cone-beam CTA for postprocedural
monitoring. Given the unpredictable nature of mycotic aneurysms and the weak correlation between size and
rupture risk, surgical or endovascular treatment should
be considered for unruptured aneurysms that enlarge
or do not regress on follow-up imaging.[7] [14] Ruptured or
symptomatic mycotic aneurysms also require surgical
or endovascular intervention.[14] A surgical approach
is indicated when an aneurysm exerts mass effect[14] or
supplies an eloquent brain region.[1] However, clipping
may be difficult due to a wide or absent aneurysmal neck
and fragile vessels.[3]
An endovascular approach is indicated for those unfit for
surgery due to cardiac disease.[3] It can be divided into
direct or indirect approaches. An indirect approach with
parent artery occlusion is the endovascular treatment of
choice, especially for distally located aneurysms and
circumferential vessel involvement. However, parent
artery sacrifice is not possible at times and the direct
approach may remain the only viable option. The direct approach using coils or liquid embolic agents allows
precise control of the aneurysm while preserving distal
flow from the parent artery. Endovascular coiling may be
a safer option with higher occlusion and lower procedure-related
complication rates[7] (Figure 7). Detachable
coils allow precise deployment and better durability
compared with liquid embolic agents. They are preferred
in proximal aneurysms, while liquid embolic agents
are more suitable for distal aneurysms not accessible
by microcatheter. Intracranial flow diverters can be
used to divert turbulent blood flow from the aneurysm
and preserve laminar blood flow in the main vessel
and its side branches. With reduced blood flow to the
aneurysm and gradual vessel remodelling, this results
in progressive aneurysmal sac thrombosis[16] (Figure 8). It is important to note that mycotic aneurysms may
grow after simple coiling, while the parent artery may
thrombose after flow diverter placement in the setting
of infection.
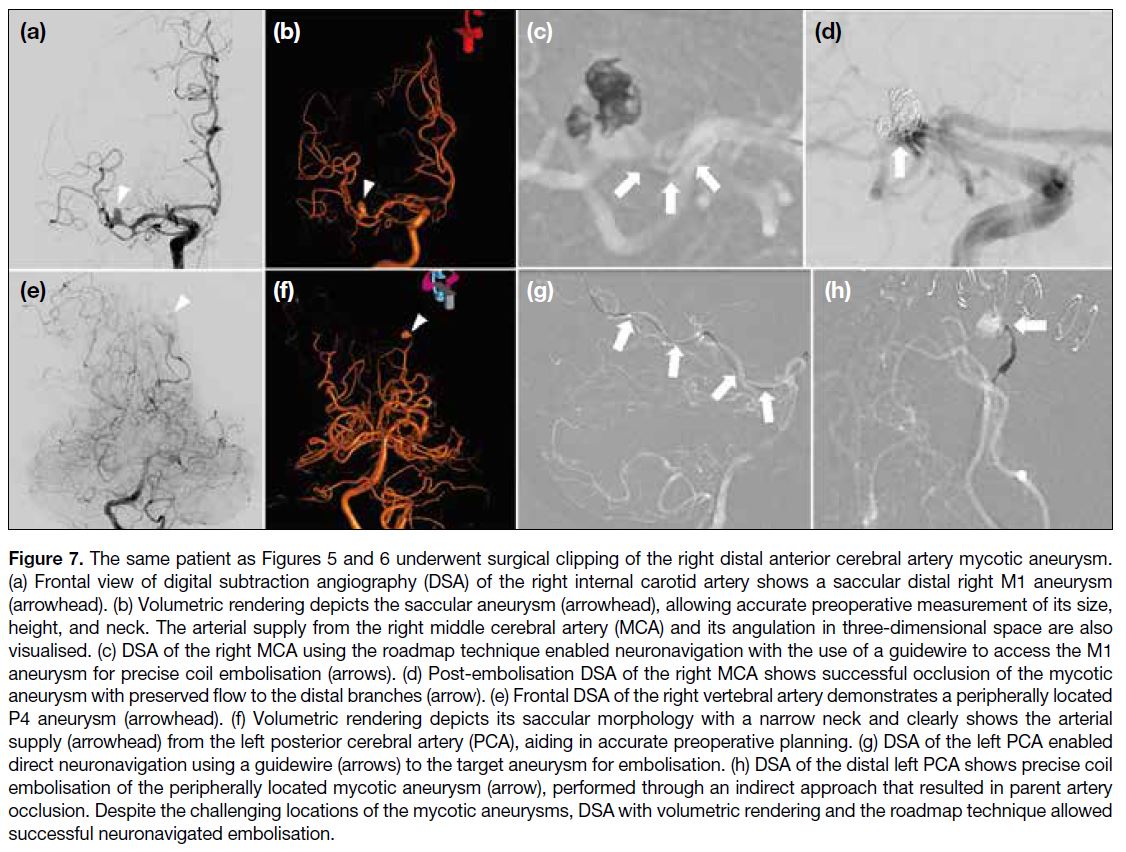
Figure 7. The same patient as Figures 5 and 6 underwent surgical clipping of the right distal anterior cerebral artery mycotic aneurysm.
(a) Frontal view of digital subtraction angiography (DSA) of the right internal carotid artery shows a saccular distal right M1 aneurysm
(arrowhead). (b) Volumetric rendering depicts the saccular aneurysm (arrowhead), allowing accurate preoperative measurement of its size,
height, and neck. The arterial supply from the right middle cerebral artery (MCA) and its angulation in three-dimensional space are also
visualised. (c) DSA of the right MCA using the roadmap technique enabled neuronavigation with the use of a guidewire to access the M1
aneurysm for precise coil embolisation (arrows). (d) Post-embolisation DSA of the right MCA shows successful occlusion of the mycotic
aneurysm with preserved flow to the distal branches (arrow). (e) Frontal DSA of the right vertebral artery demonstrates a peripherally located
P4 aneurysm (arrowhead). (f) Volumetric rendering depicts its saccular morphology with a narrow neck and clearly shows the arterial
supply (arrowhead) from the left posterior cerebral artery (PCA), aiding in accurate preoperative planning. (g) DSA of the left PCA enabled
direct neuronavigation using a guidewire (arrows) to the target aneurysm for embolisation. (h) DSA of the distal left PCA shows precise coil
embolisation of the peripherally located mycotic aneurysm (arrow), performed through an indirect approach that resulted in parent artery
occlusion. Despite the challenging locations of the mycotic aneurysms, DSA with volumetric rendering and the roadmap technique allowed
successful neuronavigated embolisation.
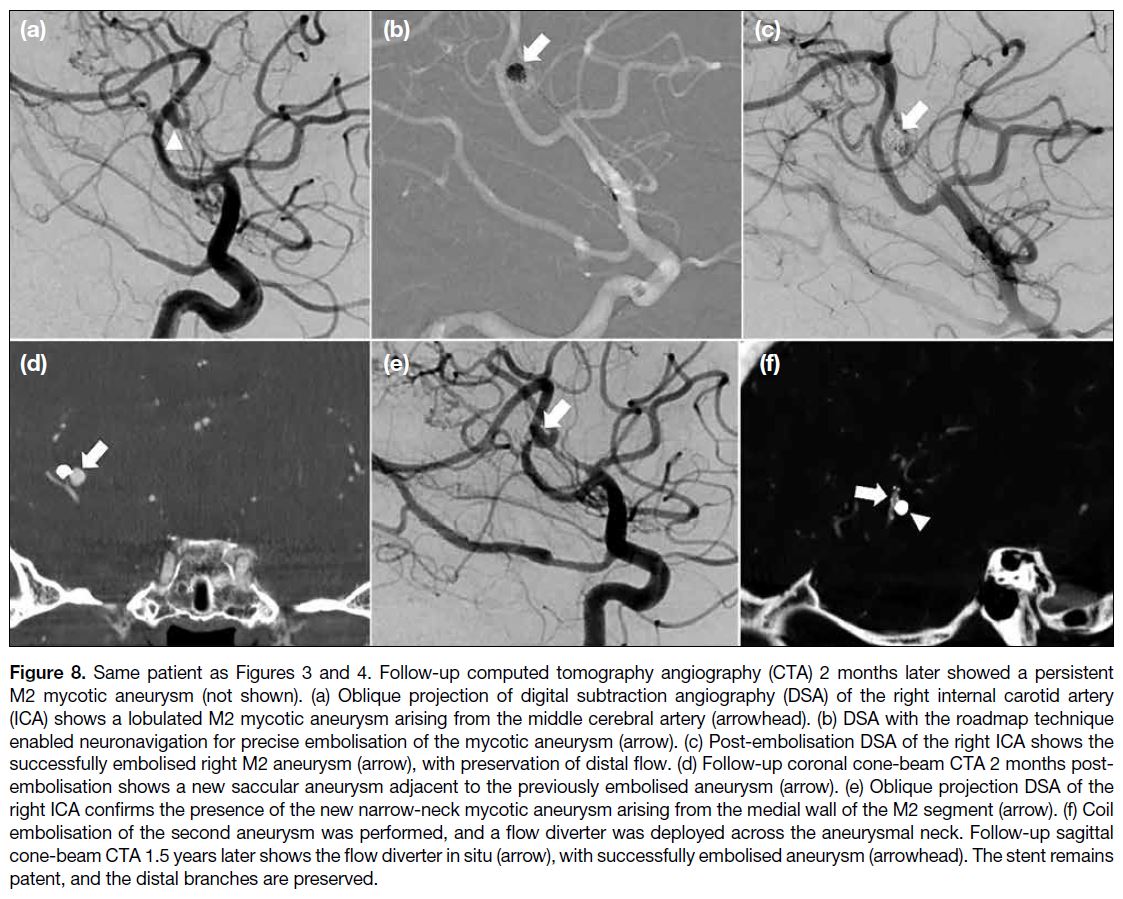
Figure 8. Same patient as Figures 3 and 4. Follow-up computed tomography angiography (CTA) 2 months later showed a persistent
M2 mycotic aneurysm (not shown). (a) Oblique projection of digital subtraction angiography (DSA) of the right internal carotid artery
(ICA) shows a lobulated M2 mycotic aneurysm arising from the middle cerebral artery (arrowhead). (b) DSA with the roadmap technique
enabled neuronavigation for precise embolisation of the mycotic aneurysm (arrow). (c) Post-embolisation DSA of the right ICA shows the
successfully embolised right M2 aneurysm (arrow), with preservation of distal flow. (d) Follow-up coronal cone-beam CTA 2 months post-embolisation
shows a new saccular aneurysm adjacent to the previously embolised aneurysm (arrow). (e) Oblique projection DSA of the
right ICA confirms the presence of the new narrow-neck mycotic aneurysm arising from the medial wall of the M2 segment (arrow). (f) Coil
embolisation of the second aneurysm was performed, and a flow diverter was deployed across the aneurysmal neck. Follow-up sagittal
cone-beam CTA 1.5 years later shows the flow diverter in situ (arrow), with successfully embolised aneurysm (arrowhead). The stent remains
patent, and the distal branches are preserved.
CONCLUSION
Neurological complications secondary to IE require
prompt recognition of its typical presentations and
imaging manifestations to facilitate early diagnosis
of neurological complications and their subsequent
treatment, including possible radiological intervention.
REFERENCES
1. Ferro JM, Fonseca AC. Infective endocarditis. Handb Clin Neurol. 2014;119:75-91. Crossref
2. Papadimitriou-Olivgeris M, Guery B, Ianculescu N, Dunet V,
Messaoudi Y, Pistocchi S, et al. Role of cerebral imaging on
diagnosis and management in patients with suspected infective
endocarditis. Clin Infect Dis. 2023;77:371-9. Crossref
3. Morris NA, Matiello M, Lyons JL, Samuels MA. Neurologic
complications in infective endocarditis: identification, management,
and impact on cardiac surgery. Neurohospitalist. 2014;4:213-22. Crossref
4. Hess A, Klein I, Iung B, Lavallée P, Ilic-Habensus E, Dornic Q, et al.
Brain MRI findings in neurologically asymptomatic patients with
infective endocarditis. AJNR Am J Neuroradiol. 2013;34:1579-84. Crossref
5. Duval X, Iung B, Klein I, Brochet E, Thabut G, Arnoult F, et al.
Effect of early cerebral magnetic resonance imaging on clinical
decisions in infective endocarditis: a prospective study. Ann Intern
Med. 2010;152:497-504, W175. Crossref
6. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP,
Del Zotti F, et al. 2015 ESC Guidelines for the management of
infective endocarditis: The Task Force for the Management of
Infective Endocarditis of the European Society of Cardiology
(ESC). Endorsed by: European Association for Cardio-Thoracic
Surgery (EACTS), the European Association of Nuclear Medicine
(EANM). Eur Heart J. 2015;36:3075-128. Crossref
7. Zanaty M, Chalouhi N, Starke RM, Tjoumakaris S, Gonzalez LF, Hasan D, et al. Endovascular treatment of cerebral mycotic
aneurysm: a review of the literature and single center experience.
Biomed Res Int. 2013;2013:151643. Crossref
8. Ahn Y, Joo L, Suh CH, Kim S, Shim WH, Kim SJ, et al. Impact
of brain MRI on the diagnosis of infective endocarditis and
treatment decisions: systematic review and meta-analysis. AJR
Am J Roentgenol. 2022;218:958-68. Crossref
9. Zakhari N, Castillo M, Torres C. Unusual cerebral emboli. Neuroimaging Clin N Am. 2016;26:147-63. Crossref
10. Allen LM, Hasso AN, Handwerker J, Farid H. Sequence-specific
MR imaging findings that are useful in dating ischemic stroke.
Radiographics. 2012;32:1285-97. Crossref
11. Cho IJ, Kim JS, Chang HJ, Kim YJ, Lee SC, Choi JH, et al.
Prediction of hemorrhagic transformation following embolic
stroke in patients with prosthetic valve endocarditis. J Cardiovasc
Ultrasound. 2013;21:123-9. Crossref
12. Chapot R, Houdart E, Saint-Maurice JP, Aymard A, Mounayer C, Lot G, et al. Endovascular treatment of cerebral mycotic aneurysms.
Radiology. 2002;222:389-96. Crossref
13. Hui FK, Bain M, Obuchowski NA, Gordon S, Spiotta AM,
Moskowitz S, et al. Mycotic aneurysm detection rates with cerebral
angiography in patients with infective endocarditis. J Neurointerv
Surg. 2015;7:449-52. Crossref
14. Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious
aneurysm): current options for diagnosis and management.
Neurocrit Care. 2009;11:120-9. Crossref
15. Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R,
Kotilainen P. Neurologic manifestations of infective endocarditis:
a 17-year experience in a teaching hospital in Finland. Arch Intern
Med. 2000;160:2781-7. Crossref
16. Ravindran K, Casabella AM, Cebral J, Brinjikji W, Kallmes DF,
Kadirvel R. Mechanism of action and biology of flow diverters
in the treatment of intracranial aneurysms. Neurosurgery.
2020;86(Suppl 1):S13-9. Crossref