Vascular Abnormalities in the Breast: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2025;28:Epub 10 December 2025
Vascular Abnormalities in the Breast: A Pictorial Essay
SH Lee, S Yang, GHC Wong
Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong SAR, China
Correspondence: Dr SH Lee, Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong SAR, China. Email: lsh689@ha.org.hk
Submitted: 15 October 2024; Accepted: 5 February 2025. This version may differ from the final version when published in an issue.
Contributors: SHL designed the study. SHL and GHCW acquired the data. SHL and SY analysed the data. SHL draft the manuscript. SY
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2024-186-2). A waiver of patient consent and acknowledgment forms was approved by the Board due to the retrospective nature of the study.
INTRODUCTION
Vascular lesions are not uncommonly seen in the
breast. They can range from benign haemangiomas
to aggressive angiosarcomas. Benign lesions and
vascular malformations are usually asymptomatic when
small in size and may only be incidentally found on
imaging. Malignant angiosarcomas are extremely rare
and aggressive, often presenting with disseminated
metastases on diagnosis. This pictorial essay aims to
illustrate the common imaging features of vascular
lesions, with the cases identified in the database of a
local tertiary hospital in Hong Kong from 2008 to 2024.
BENIGN VASCULAR LESIONS
Haemangioma
Haemangiomas are commonly found in the hepatobiliary
and musculoskeletal systems, as well as in the breasts,
where they are usually small in size and asymptomatic,
usually presenting as an incidental imaging finding. It
has been reported that haemangiomas are found in 1.2%
of mastectomy specimens and 11% of post-mortem
specimens (from a forensic population) of the female
breast.[1] Some patients might present with blue skin
discolouration when the lesion is large and superficial.
On mammography, haemangiomas are usually equal
dense to the breast tissue, and oval in shape with
circumscribed or microlobulated margins (Figure 1).[2] Intralesional microcalcifications may be found
occasionally[2], which may lead to the need for imaging
surveillance or core biopsy.
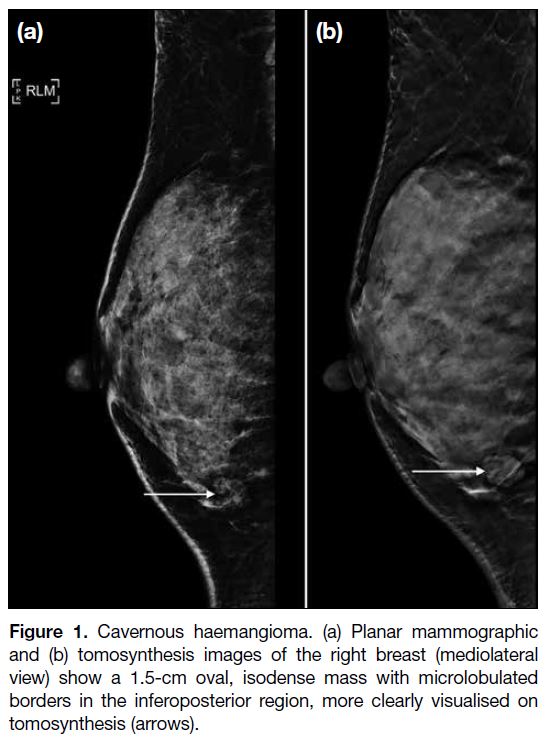
Figure 1. Cavernous haemangioma. (a) Planar mammographic
and (b) tomosynthesis images of the right breast (mediolateral
view) show a 1.5-cm oval, isodense mass with microlobulated
borders in the inferoposterior region, more clearly visualised on
tomosynthesis (arrows).
Sonographically, they are similar to their shape on
mammography and are oriented in a parallel manner
(Figures 2 and 3).[2] Their echogenicity is variable,[2] and
they may exhibit non-specific vascular flow.[3] Overall,
there are no definitive imaging features to suggest
benignity or malignancy. Histologically, these lesions
may present as a proliferation of variably sized, ectatic
blood vessels separated by fibrotic stroma.
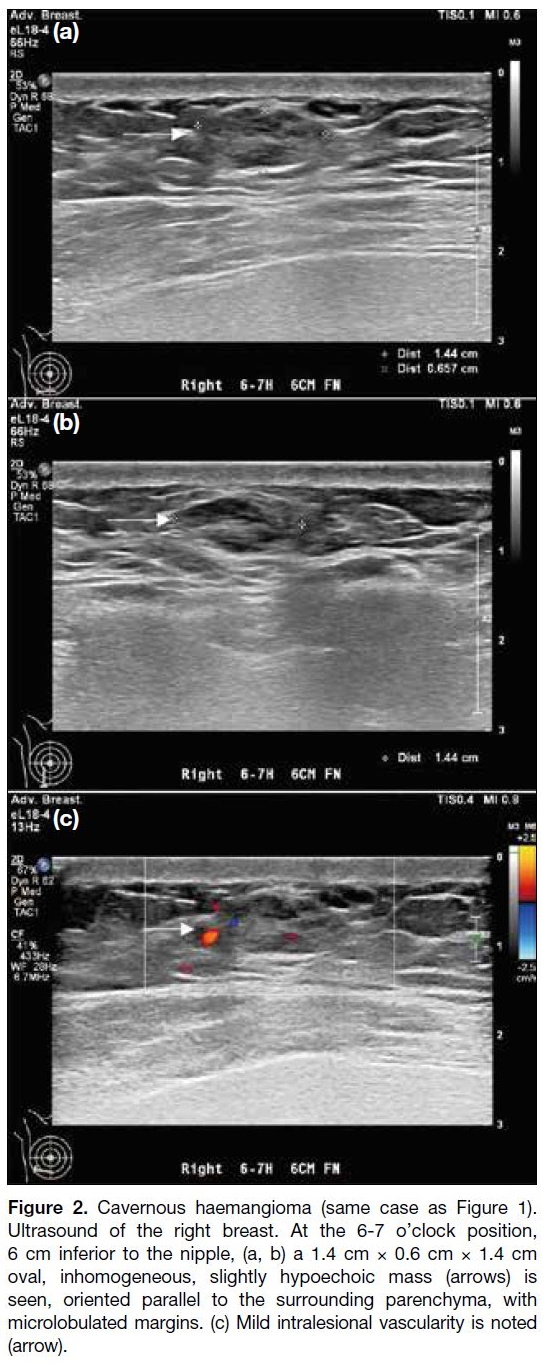
Figure 2. Cavernous haemangioma (same case as Figure 1). Ultrasound of the right breast. At the 6-7 o’clock position, 6 cm inferior to the nipple, (a, b) a 1.4 cm × 0.6 cm × 1.4 cm oval, inhomogeneous, slightly hypoechoic mass (arrows) is seen, oriented parallel to the surrounding parenchyma, with microlobulated margins. (c) Mild intralesional vascularity is noted (arrow).
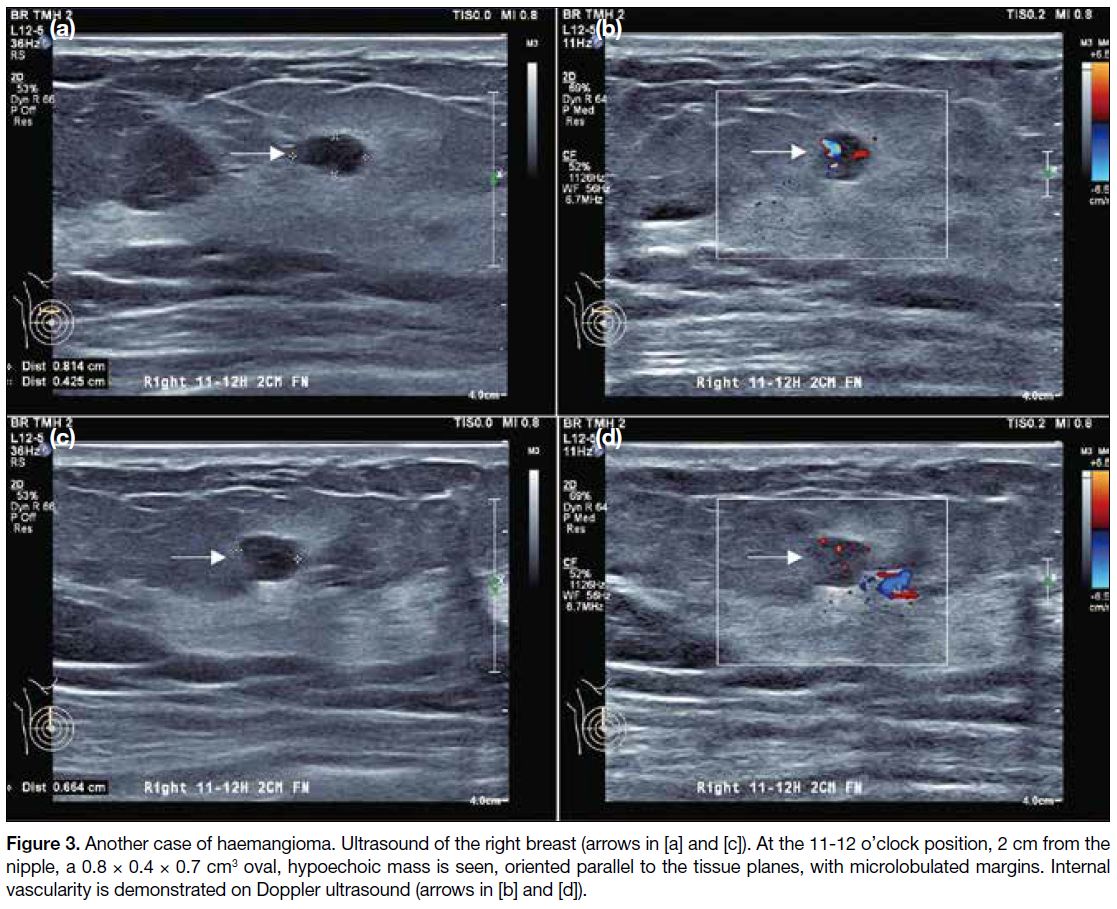
Figure 3. Another case of haemangioma. Ultrasound of the right breast arrows in [a] and [c]. At the 11-12 o’clock position, 2 cm from the nipple, a 0.8
× 0.4 × 0.7 cm3 oval, hypoechoic mass is seen, oriented parallel to the tissue planes, with microlobulated margins. Internal vascularity is
demonstrated on Doppler ultrasound (arrows in [b] and [d]).
Management of benign haemangiomas remains
controversial due to the sampling error of core biopsy
samples and difficulty in clearly delineating the borders
of the haemangiomas confidently on core samples. A
recent review had explored the possibility of clinical
and radiological surveillance in cases of radiologically
pathologically concordant haemangiomas, but surgical
excision remains the mainstay of management.[4]
Superficial Venous Thrombophlebitis:
Mondor’s Disease
Mondor’s disease is a rare disease characterised by
inflammation and thrombosis of the superficial venous
structures in the breast. This disease is found in less
than 1% of the population.[5] Clinically, patients present
with skin swelling or tender cord-like palpable masses.
On mammography, elongated equal density tubular
structures are usually found in the upper outer quadrant
where the lateral thoracic veins are located (Figure 4).[6]
Ultrasound should be performed to exclude underlying
breast malignancy since dilated ducts could mimic
Mondor’s disease mammographically.[6]
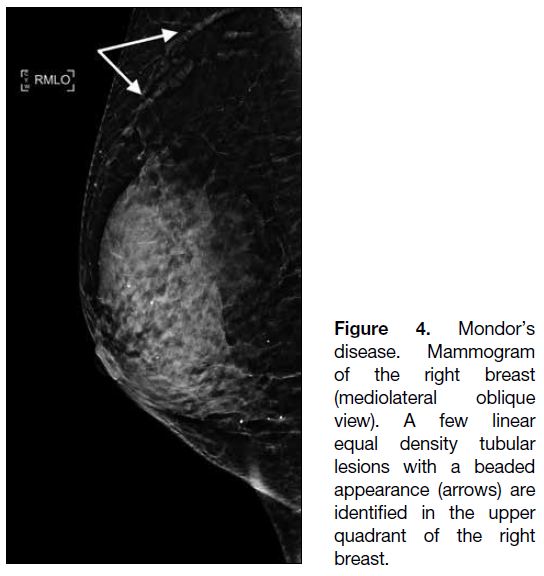
Figure 4. Mondor’s
disease. Mammogram
of the right breast
(mediolateral oblique
view). A few linear
equal density tubular
lesions with a beaded
appearance (arrows) are
identified in the upper
quadrant of the right
breast.
On ultrasound, Mondor’s disease is seen as a tubular
hypoechoic structure with a beaded appearance, which
is the classical finding of a thrombosed vein, but not of
a dilated duct which usually with smooth wall (Figure 5).[6] It is also longer in extent and will not be connected
to the nipple areolar complex.[6] Similar to venous
thrombosis in the body elsewhere, the distended vein is
not compressible by the ultrasound probe and there is
absence of Doppler signal.[5]
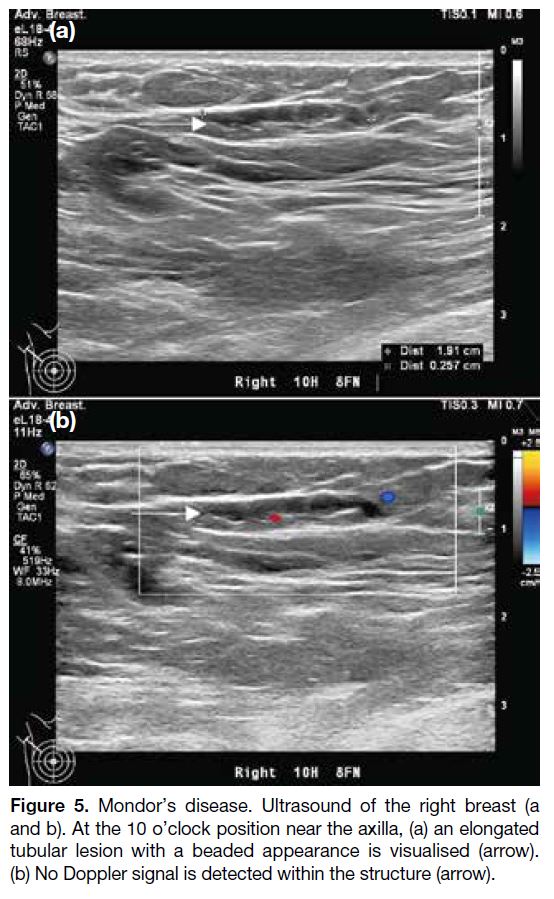
Figure 5. Mondor’s disease. Ultrasound of the right breast (a and
b). At the 10 o’clock position near the axilla, (a) an elongated tubular
lesion with a beaded appearance is visualised (arrow). (b) No
Doppler signal is detected within the structure (arrow).
Mondor’s disease does not require a pathological
diagnosis when clinical and radiological findings
are concordant. No specific treatment is needed for
the disease since it will resolve spontaneously in 1 to 2 months’ time.[5] Nonsteroidal anti-inflammatory
drugs may be considered in symptomatic cases if not
contraindicated.[5]
VASCULAR MALFORMATIONS
High Flow: Arteriovenous Malformation
Arteriovenous malformations (AVMs) are exceedingly
rare in the breast, with only scant case reports. No specific
mammographic features are found in AVMs. Similar
to other benign vascular entities, they may present on
mammography as an equal density mass with a round
shape and circumscribed margins (Figure 6). They may
also contain benign-appearing calcifications, indicating
the presence of phleboliths.[7]
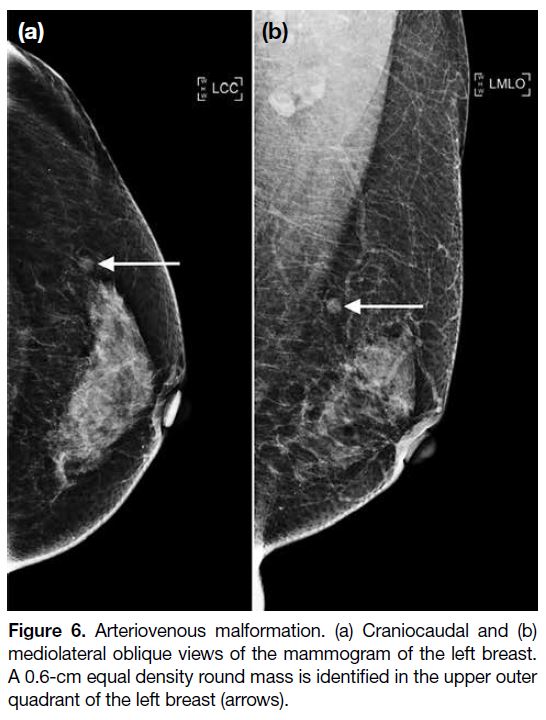
Figure 6. Arteriovenous malformation. (a) Craniocaudal and (b)
mediolateral oblique views of the mammogram of the left breast.
A 0.6-cm equal density round mass is identified in the upper outer
quadrant of the left breast (arrows).
On ultrasound, they are again non-specific. In our case,
the lesion presented as an oval heterogeneous hypoechoic
mass with parallel orientation to the skin surface. No
posterior acoustic enhancement was demonstrated. Doppler ultrasound may be a non-invasive technique
to establish the diagnosis since it can demonstrate the
mixture of arterial and venous blood flow within the
lesion (Figure 7).[8]
Figure 7. Arteriovenous malformation. Ultrasound of the left breast. At the 2 o’clock position, 3 cm from the nipple, a 0.6 × 0.4 × 0.5 cm3 oval, heterogeneous, hypoechoic mass is visualised with parallel orientation and no posterior acoustic shadowing. It demonstrates
internal arterial and venous flow, with communication to adjacent breast parenchymal vasculature (arrows).
Magnetic resonance imaging (MRI) can demonstrate a
tangle of dilated blood vessels with progressive contrast
enhancement of the lesion.[9] Blooming artefacts indicate
the presence of phleboliths.[9]
The management of AVMs is dependent on the clinical
symptoms. For asymptomatic and small masses, as in our
case, conservative management should be considered. In
case of palpable symptomatic lesions, embolisation or
surgical excision would be the options.[9]
Slow Flow: Venous and Venolymphatic
Malformations
Venous and venolymphatic malformations are slow-flow
vascular lesions, which may be asymptomatic.
Some patients present with bluish skin discolouration
and even with enlarged breast volume when the lesion
is more sizable.
Ultrasound can be a good initial tool to establish the
diagnosis. These lesions are identified as an area of
hypoechogenicity within the breast. Doppler ultrasound
on this hypoechoic area will demonstrate the venous
flow pattern on spectral technique (Figure 8). Other
sonographic findings include echogenic foci indicating
phleboliths and absence of colour uptake due to
thrombosis or lymphatic components.[10]
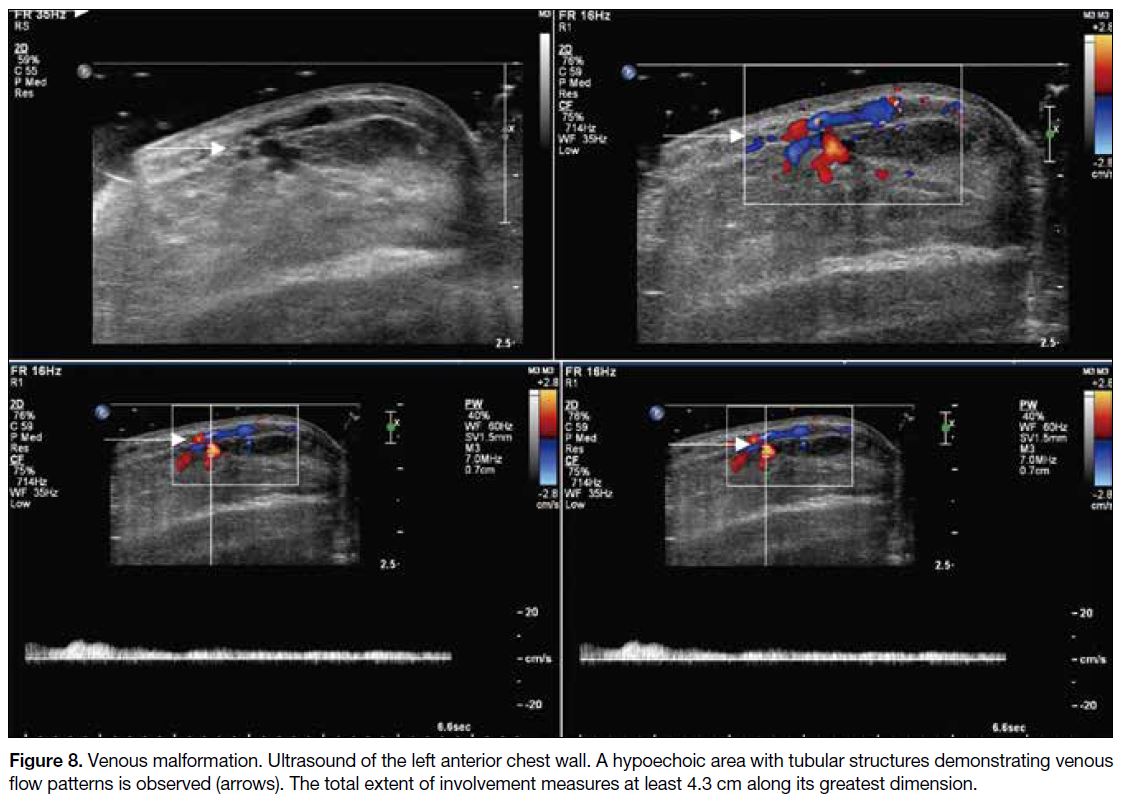
Figure 8. Venous malformation. Ultrasound of the left anterior chest wall. A hypoechoic area with tubular structures demonstrating
venous flow patterns is observed (arrows). The total extent of involvement measures at least 4.3 cm along its greatest dimension.
Venous malformations can present as T2-weighted
hyperintense tubular structures. Variable unenhanced
T1-weighted signal has been described depending
on whether these structures contain thrombosis.[10]
Enhancement could be seen on T1-weighted images
after gadolinium injection (Figure 9). If phleboliths
are present, susceptibility artefacts may also be seen.[11]
For the lymphatic component, MRI might demonstrate
septal enhancement in the background of non-enhancing
lymphatic fluid.[12]
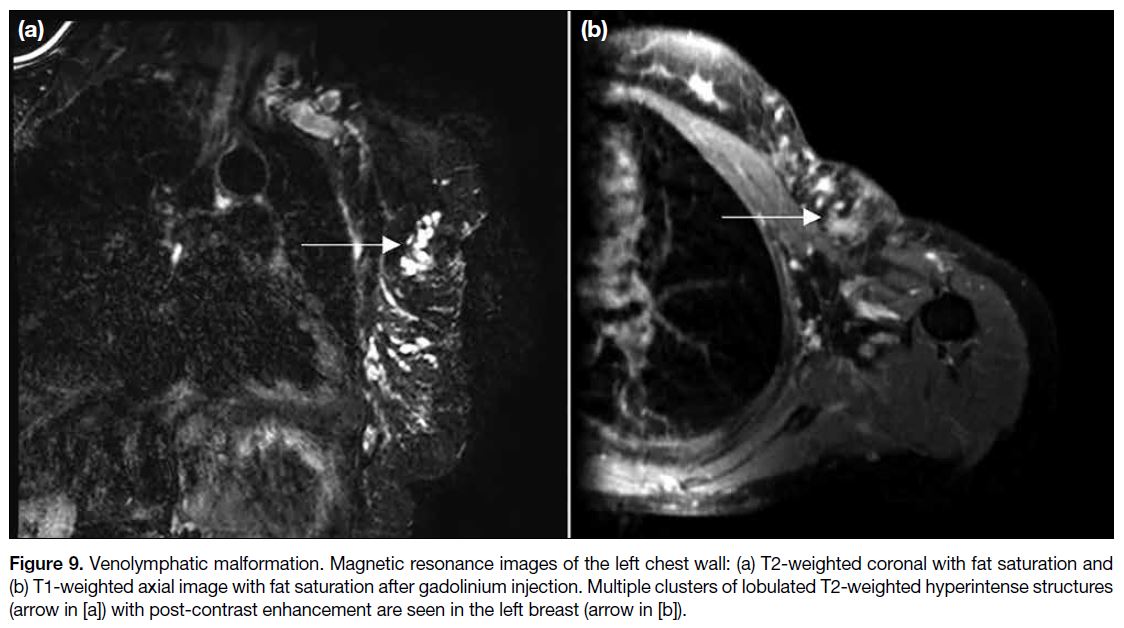
Figure 9. Venolymphatic malformation. Magnetic resonance images of the left chest wall: (a) T2-weighted coronal with fat saturation and (b) T1-weighted axial image with fat saturation after gadolinium injection. Multiple clusters of lobulated T2-weighted hyperintense structures (arrow in [a]) with post-contrast enhancement are seen in the left breast (arrow in [b]).
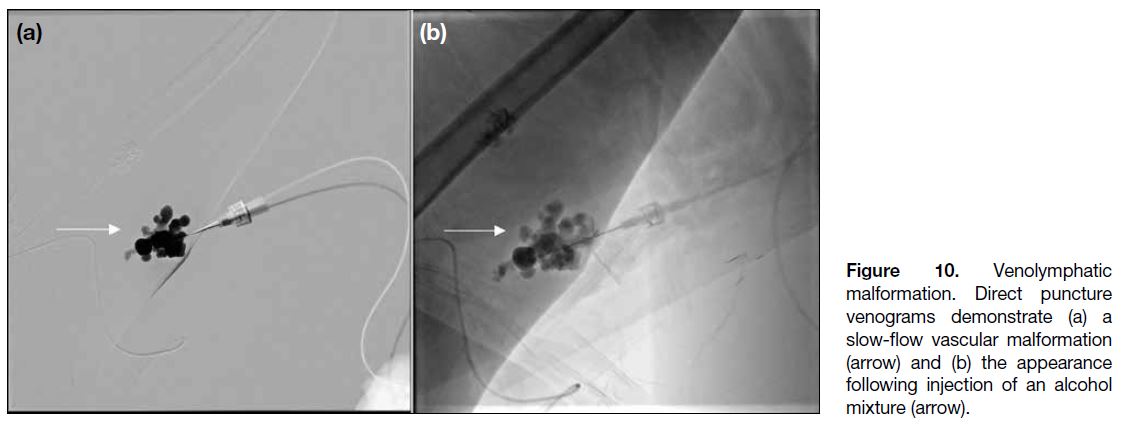
If patients are symptomatic or there is cosmetic concern,
sclerotherapy, laser therapy, or surgical resection
are options.[11] In our case, sclerotherapy with sodium
tetradecyl sulphate foam was performed under the
guidance of direct puncture venography (Figure 10),
with resulting clinical improvement.
Figure 10. Venolymphatic malformation. Direct puncture venograms demonstrate (a) a slow-flow vascular malformation (arrow) and (b) the appearance following injection of an alcohol mixture (arrow).
MALIGNANT VASCULAR LESIONS
Angiosarcoma
Angiosarcoma is an exceedingly rare cause of primary
breast tumour, with the literature-quoted incidence
rate <0.05%.13 There are no specific mammographic
findings.[13]
On ultrasound, they show a variable echogenic pattern,
usually with hypervascularity on Doppler.[13] However,
as mentioned previously, haemangiomas may also
demonstrate hypervascularity, so this feature is not
specific.
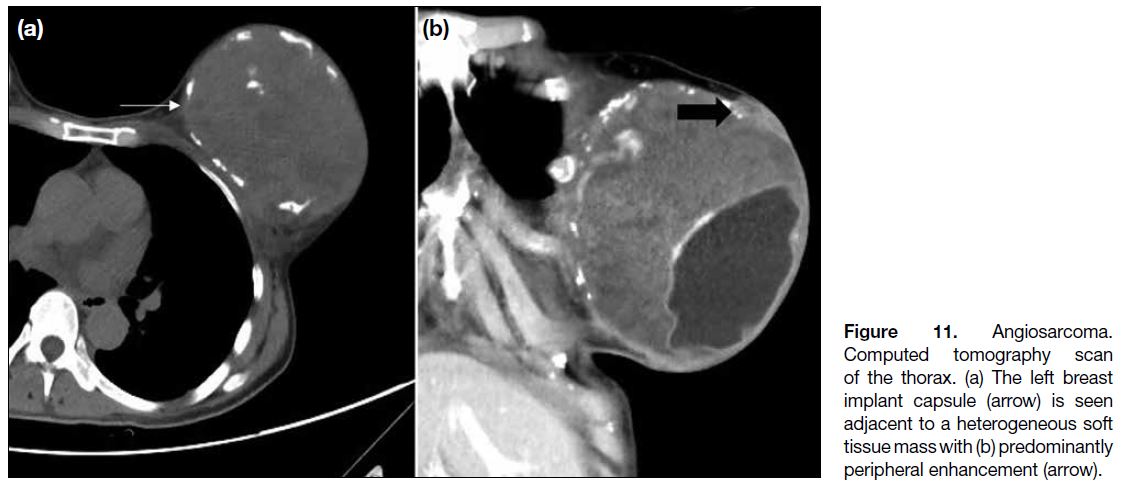
In our case, similar to other breast tumours, angiosarcomas
may enhance after contrast administration on computed
tomography scan (Figure 11).
Figure 11. Angiosarcoma.
Computed tomography scan of
the thorax. (a) The left breast implant
capsule (arrow) is seen
adjacent to a heterogeneous soft
tissue mass with (b) predominantly
peripheral enhancement (arrow).
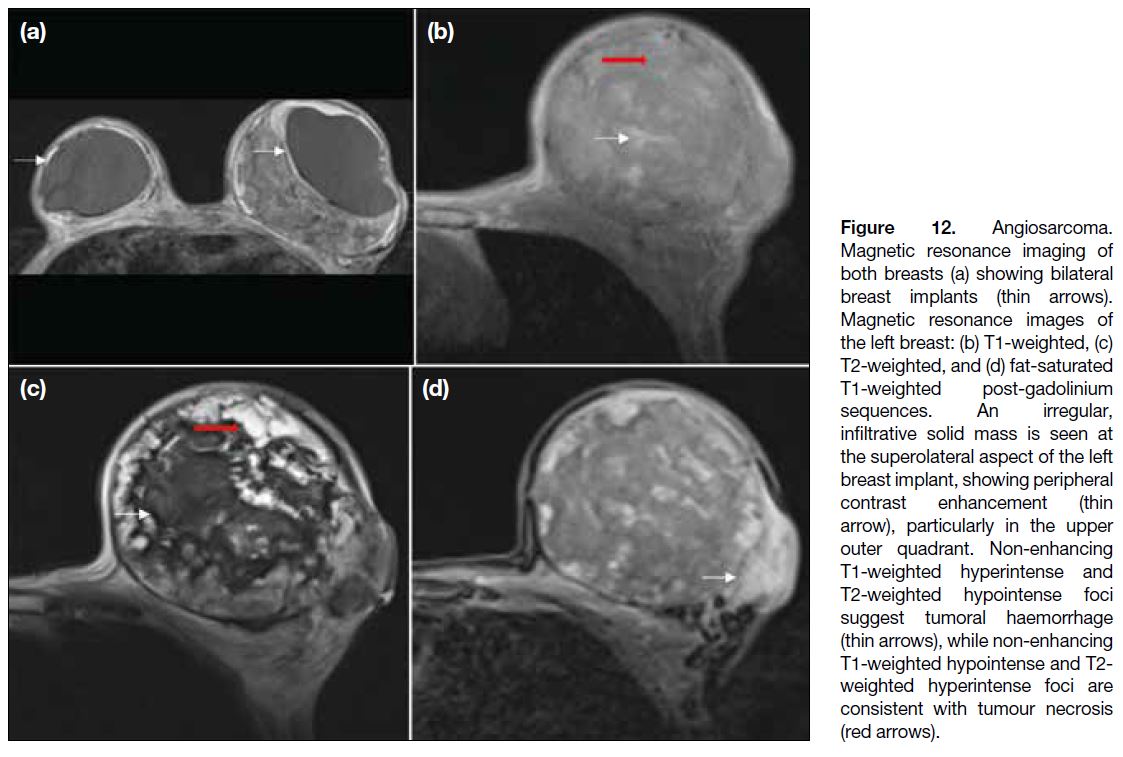
On MRI scan, they showed variable signal intensity
on T1-weighted and T2-weighted images. High T1-weighted signal suggests the presence of haemorrhagic
products or venous lakes.[13] Elevated T2-weighted signals indicate the aggressiveness of the lesion
with cystic degeneration and tumour necrosis.[14]
They enhance, often at the periphery only, after
gadolinium administration (Figure 12).[14] The kinetic
characteristics of the tumours depend on their
grade.[13]
Figure 12. Angiosarcoma.
Magnetic resonance imaging of
both breasts (a) showing bilateral
breast implants (thin arrows).
Magnetic resonance images of
the left breast: (b) T1-weighted, (c)
T2-weighted, and (d) fat-saturated
T1-weighted post-gadolinium
sequences. An irregular,
infiltrative solid mass is seen at
the superolateral aspect of the left
breast implant, showing peripheral
contrast enhancement (thin
arrow), particularly in the upper
outer quadrant. Non-enhancing
T1-weighted hyperintense and
T2-weighted hypointense foci
suggest tumoral haemorrhage
(thin arrows), while non-enhancing
T1-weighted hypointense and T2-weighted hyperintense foci are
consistent with tumour necrosis
(red arrows).
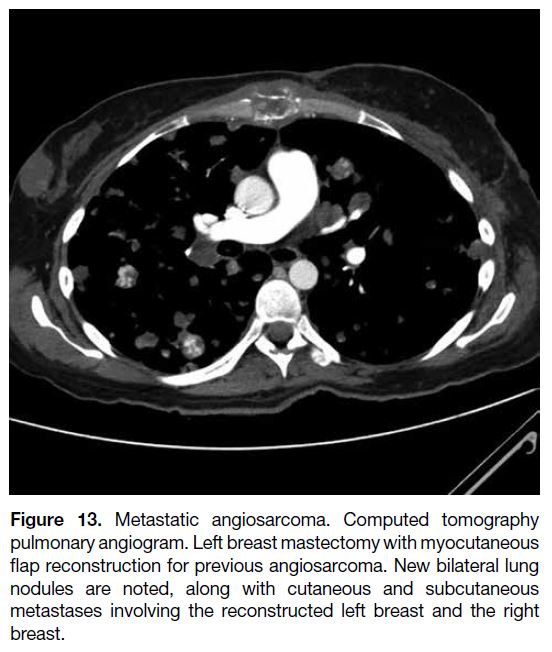
Aggressive surgery remains the mainstay of treatment,
while there is still no consensus on adjuvant treatment.[15]
Angiosarcomas carry a poor prognosis, with the
majority of the cases in our centre showing disseminated
hypervascular metastases or contralateral breast
metastases despite radical surgery performed after
diagnosis (Figure 13).
Figure 13. Metastatic angiosarcoma. Computed tomography
pulmonary angiogram. Left breast mastectomy with myocutaneous
flap reconstruction for previous angiosarcoma. New bilateral lung
nodules are noted, along with cutaneous and subcutaneous
metastases involving the reconstructed left breast and the right
breast.
CONCLUSION
Vascular lesions have been increasingly discovered due
to increased health screening. We should be aware of
the typical imaging features of vascular malformations
to avoid unnecessary biopsies. While histopathological
results are still required to establish the diagnosis in
haemangiomas and angiosarcoma—with surgical
excision remaining the mainstay of management—if the
imaging features of haemangiomas are benign, such as
oval/lobulated shape with well-circumscribed margins,
imaging surveillance could be performed after needle
biopsy.[2]
REFERENCES
1. Kim KM, Kim JY, Kim SH, Jeong MJ, Kim SH, Kim JH,
et al. Breast cavernous hemangioma with increased size on
ultrasonography: a case report. J Korean Soc Radiol. 2018;79:311-4. Crossref
2. Mesurolle B, Sygal V, Lalonde L, Lisbona A, Dufresne MP,
Gagnon JH, et al. Sonographic and mammographic appearances
of breast hemangioma. AJR Am J Roentgenol. 2008;191:W17-22. Crossref
3. Glazebrook KN, Morton MJ, Reynolds C. Vascular tumors of the breast: mammographic, sonographic, and MRI appearances. AJR Am J Roentgenol. 2005;184:331-8. Crossref
4. Azam R, Mrkonjic M, Gupta A, Gladdy R, Covelli AM.
Mesenchymal tumors of the breast: fibroblastic/myofibroblastic
lesions and other lesions. Curr Oncol. 2023;30:4437-82. Crossref
5. Amano M, Shimizu T. Mondor’s disease: a review of the literature. Intern Med. 2018;57:2607-12. Crossref
6. Shetty MK, Watson AB. Mondor’s disease of the breast: sonographic and mammographic findings. AJR Am J Roentgenol.
2001;177:893-6. Crossref
7. Padmanabhan V, Dev B, Garg M, Gnanavel H. Arteriovenous malformation of the breast; report a case. Arch Breast Cancer.
2024;11:96-100. Crossref
8. O’Beirn E, Elliott J, Neary C, McLaughlin R. 32 Congenital arteriovenous malformation of the breast associated with giant
hairy naevus: a case report. Br J Surg. 2021;108(Suppl 6):znab259-64. Crossref
9. Swy E, Wahab R, Mahoney M, Vijapura C. Multimodality imaging review of breast vascular lesions. Clin Radiol. 2022;77:255-63. Crossref
10. Gautam R, Dixit R, Pradhan GS. Venous malformation in the breast: imaging features to avoid unnecessary biopsies or surgery. J Radiol Case Rep. 2023;17:1-8. Crossref
11. Vijapura C, Wahab R. Multiple venous malformations involving
the breast. J Breast Imaging. 2022;4:550-2. Crossref
12. Marín C, J Galindo P, Guzmán F, Ferri B, Parrilla P. Lymphovascular
malformation of the breast: differential diagnosis and a case report
[in English, Spanish]. Cir Esp (Engl Ed). 2019;97:541-3. Crossref
13. Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008;190:533-8. Crossref
14. Wu WH, Ji QL, Li ZZ, Wang QN, Liu SY, Yu JF. Mammography and MRI manifestations of breast angiosarcoma. BMC Womens
Health. 2019;19:73. Crossref
15. Bordoni D, Bolletta E, Falco G, Cadenelli P, Rocco N, Tessone A, et al. Primary angiosarcoma of the breast. Int J Surg Case Rep.
2016;20S(Suppl):12-5. Crossref