Solid Variant Aneurysmal Bone Cyst in Ischiopubic Ramus: A Case Report
CASE REPORT
Hong Kong J Radiol 2025;28:Epub 12 September 2025
Solid Variant Aneurysmal Bone Cyst in Ischiopubic Ramus: A Case Report
KH Chu1, WK Kung1, L Xu1, TWY Chin1, LK Tse2, JW Liao2, MK Chan1
1 Department of Diagnostic and Interventional Radiology, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Pathology, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr KH Chu, Department of Diagnostic and Interventional Radiology, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: ckh975@ha.org.hk
Submitted: 24 October 2024; Accepted: 12 February 2025. This version may differ from the final version when published in an issue.
Contributors: KHC, WKK and LKT designed the study. KHC, WKK, LKT and JWL acquired the data. All authors analysed the data, drafted
the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Central Institutional Review Board of the Hospital Authority, Hong Kong (Ref No.: IRB-2024-502). Informed consent was obtained from the patient for the study and publication of this case report.
CASE PRESENTATION
A 39-year-old male presented in December 2022 with a
fever of unknown origin. Positron emission tomography–computed tomography (PET-CT) was performed to
search for a potential source of sepsis. The patient did
not report any symptoms of pain or swelling throughout
the evaluation. Nonetheless, an incidental finding of
a bone lesion in the left ischiopubic ramus prompted
further investigation and an orthopaedic referral.
The lesion appeared subtle and poorly defined on
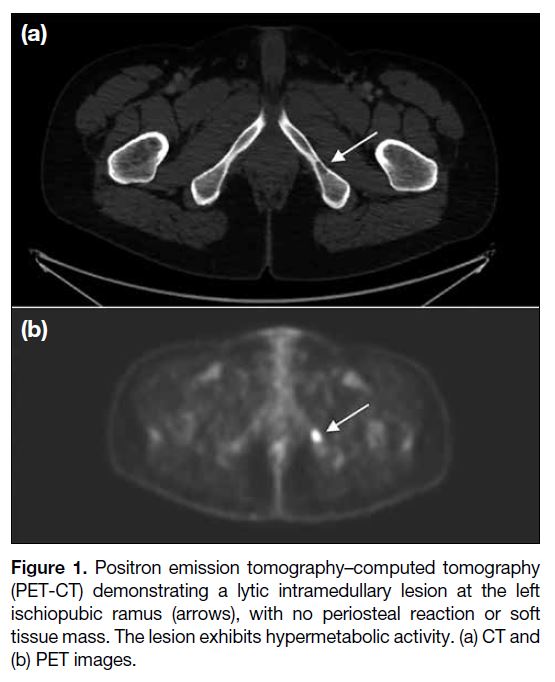
the pelvic radiograph. PET-CT revealed a lytic
intramedullary lesion in the left ischiopubic ramus, with
no periosteal reaction or soft tissue mass (Figure 1).
No pathological fracture was observed. The lesion was
hypermetabolic with a maximum standard uptake value
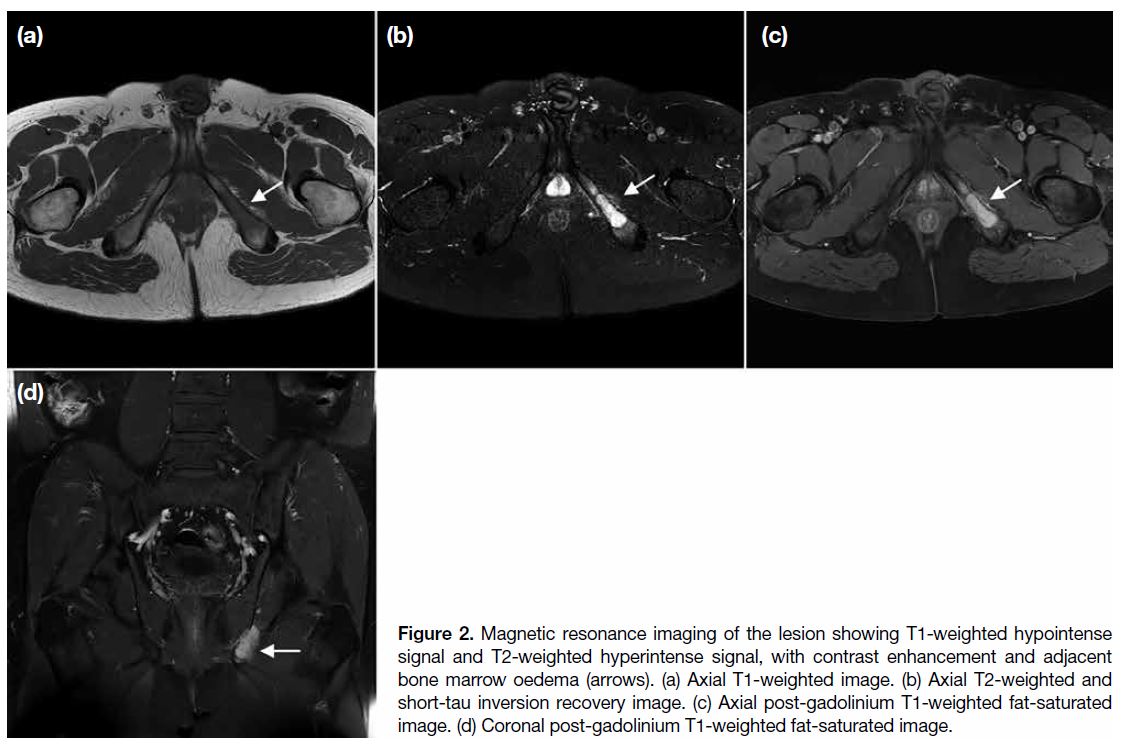
of 13.5. Magnetic resonance imaging (MRI) revealed
predominantly T1-weighted hypointense signals and T2-weighted hyperintense signals with contrast enhancement
(Figure 2). Perilesional bone marrow oedema was noted,
along with cortical disruption and adjacent soft tissue
oedema at the medial aspect of the left ischial bone.
Figure 1. Positron emission tomography–computed tomography
(PET-CT) demonstrating a lytic intramedullary lesion at the left
ischiopubic ramus (arrows), with no periosteal reaction or soft
tissue mass. The lesion exhibits hypermetabolic activity. (a) CT and
(b) PET images.
Figure 2. Magnetic resonance imaging of the lesion showing T1-weighted hypointense signal and T2-weighted hyperintense signal, with contrast enhancement and adjacent bone marrow oedema (arrows). (a) Axial T1-weighted image. (b) Axial T2-weighted and short-tau inversion recovery image. (c) Axial post-gadolinium T1-weighted fat-saturated image. (d) Coronal post-gadolinium T1-weighted fat-saturated image.
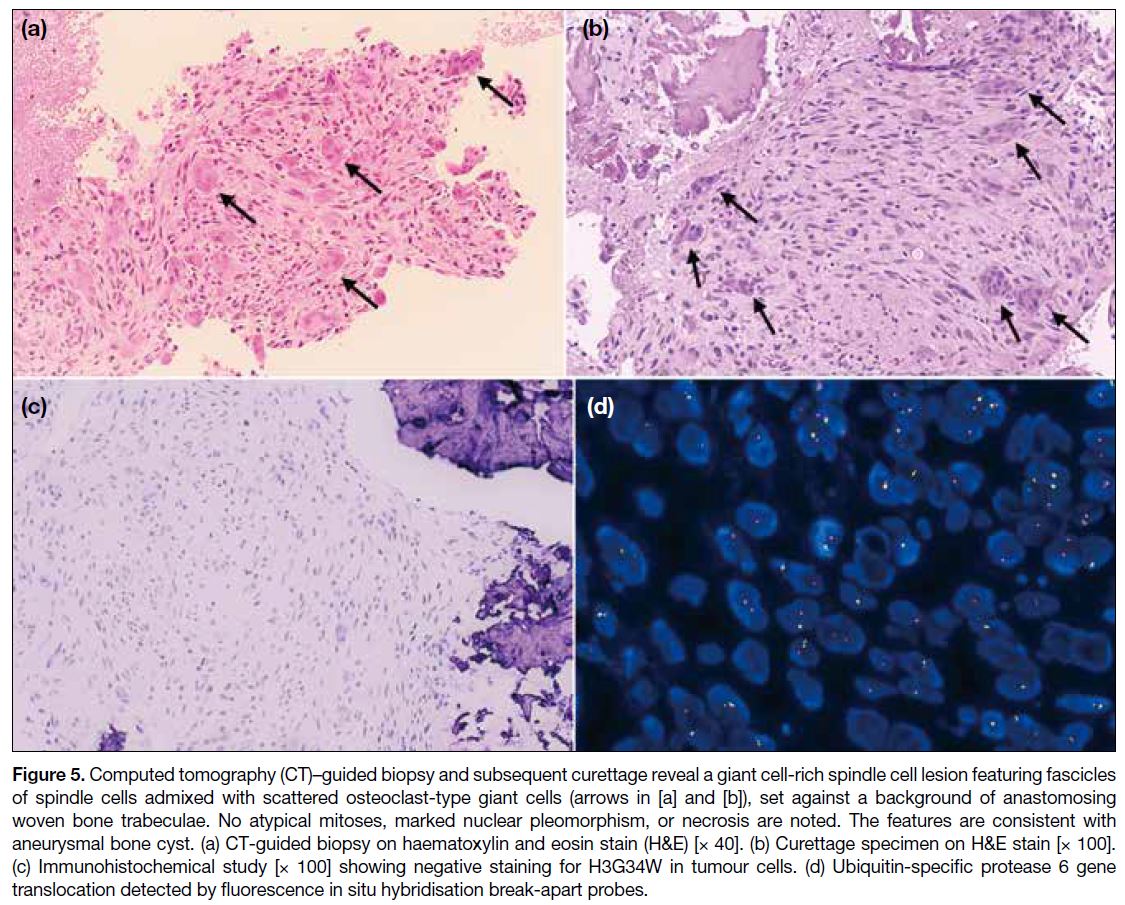
Results of the CT-guided biopsy of the lesion suggested
a giant cell-rich spindle cell lesion, most compatible
with an aneurysmal bone cyst. The biopsy showed
random fascicles of spindle cells admixed with
scattered osteoclast-type giant cells, accompanied by
occasional lymphocytes, plasma cells and haemosiderin-laden
macrophages. No atypical mitoses, marked
nuclear pleomorphism, or necrosis were noted.
Immunohistochemical study showed that the lesional
cells were negative for H3G34W and H3K36M.
Ubiquitin-specific protease 6 (USP6) gene translocation
was identified by fluorescence in situ hybridisation.
Follow-up CT and MRI were performed prior to
surgery, 2 months after the biopsy and 8 months after
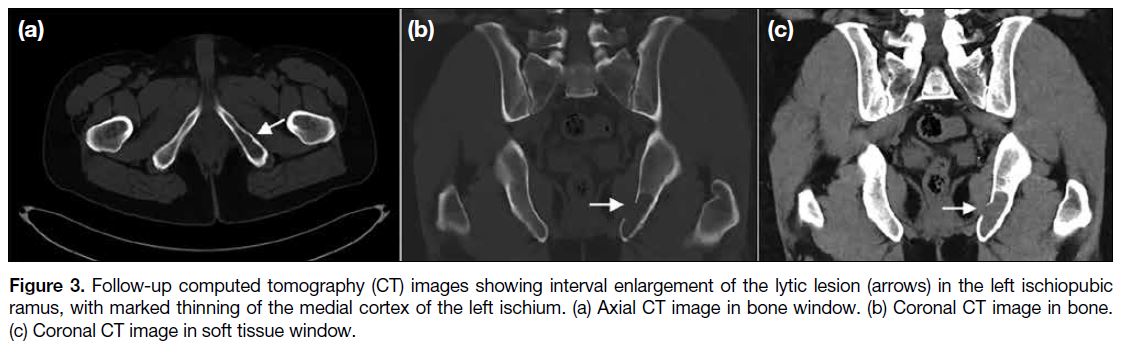
the initial imaging. The follow-up CT revealed interval
enlargement of the lesion in the left ischiopubic ramus,
with marked thinning of the medial cortex of the left
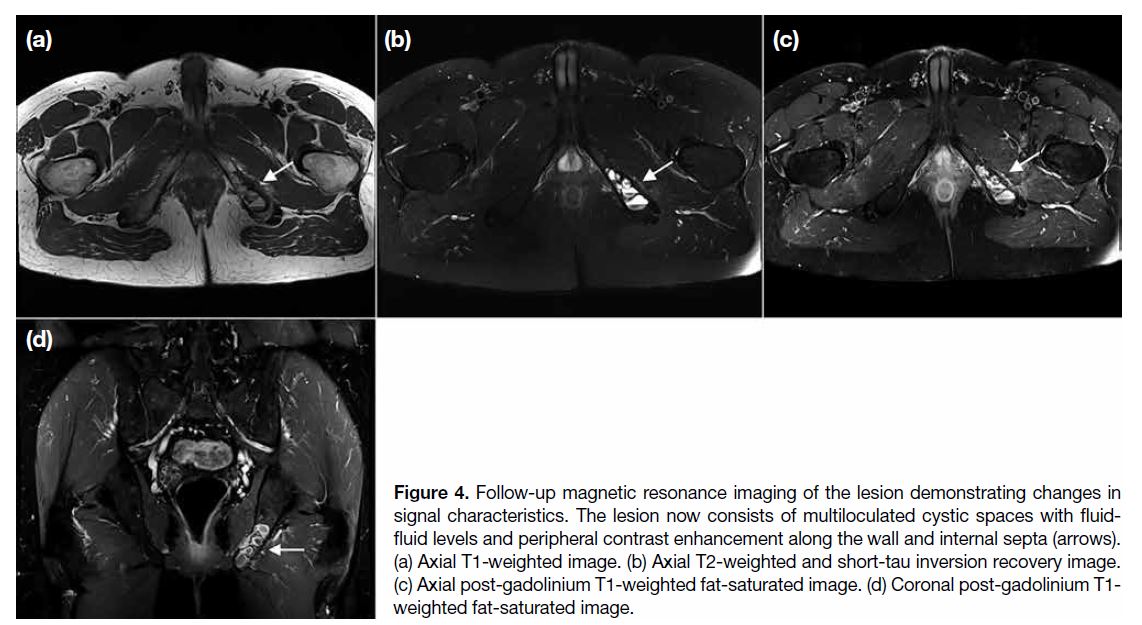
ischium (Figure 3). Follow-up MRI demonstrated
changes to the signal characteristics of the lesion, now
showing multiloculated cystic spaces with fluid-fluid
levels and peripheral contrast enhancement along
the wall and internal septa (Figure 4). There was no perilesional bone marrow oedema or extraosseous soft
tissue extension.
Figure 3. Follow-up computed tomography (CT) images showing interval enlargement of the lytic lesion (arrows) in the left ischiopubic
ramus, with marked thinning of the medial cortex of the left ischium. (a) Axial CT image in bone window. (b) Coronal CT image in bone.
(c) Coronal CT image in soft tissue window.
Figure 4. Follow-up magnetic resonance imaging of the lesion demonstrating changes in
signal characteristics. The lesion now consists of multiloculated cystic spaces with fluid-fluid levels and peripheral contrast enhancement along the wall and internal septa (arrows). (a) Axial T1-weighted image. (b) Axial T2-weighted and short-tau inversion recovery image. (c) Axial post-gadolinium T1-weighted fat-saturated image. (d) Coronal post-gadolinium T1-weighted fat-saturated image.
Subsequently, the patient underwent curettage of
the lesion, with allograft chips packed into the bone
cavities. Pathological examination revealed tumour
tissue featuring haphazardly arranged spindle cell
fascicles and osteoclast-like multinucleated giant cells,
set against a background of anastomosing woven bone
trabeculae rimmed by osteoblasts and fibrous septa
formed by bland fibroblasts (Figure 5). No cytological
atypia, increased mitosis, or necrosis were observed.
Immunohistochemical studies were once again
performed, with the lesional cells being negative for
H3G34W and H3K36M. The features were consistent
with an aneurysmal bone cyst. To date, the patient has
remained asymptomatic with no clinical evidence of
tumour recurrence.
Figure 5. Computed tomography (CT)–guided biopsy and subsequent curettage reveal a giant cell-rich spindle cell lesion featuring fascicles
of spindle cells admixed with scattered osteoclast-type giant cells (arrows in [a] and [b]), set against a background of anastomosing
woven bone trabeculae. No atypical mitoses, marked nuclear pleomorphism, or necrosis are noted. The features are consistent with
aneurysmal bone cyst. (a) CT-guided biopsy on haematoxylin and eosin stain (H&E) [× 40]. (b) Curettage specimen on H&E stain [× 100].
(c) Immunohistochemical study [× 100] showing negative staining for H3G34W in tumour cells. (d) Ubiquitin-specific protease 6 gene
translocation detected by fluorescence in situ hybridisation break-apart probes.
DISCUSSION
Conventional aneurysmal bone cyst (ABC) refers to
an expansile cystic lesion of bone composed primarily
of blood-filled spaces. Although some solid areas may be present, these are not the predominant feature.
Histologically, conventional ABC is characterised
by blood-filled cystic spaces with septa containing
fibroblasts, osteoclast-like giant cells, and reactive woven
bone.[1] The solid variant of ABC is a rare entity that
exhibits distinct radiological and pathological features.
The cystic components may be completely absent, with the
lesion demonstrating a predominantly solid architecture.
ABC was initially thought to represent a reactive and
inflammatory response to intraosseous haemorrhage.[2]
Nonetheless, with the identification of the USP6 gene
rearrangement, a translocation on chromosome 17p13,
both conventional ABC and its solid variant are now considered true bone neoplasms.[3] In the latest World
Health Organization Classification of Tumors of Bone,
ABC is classified as a benign osteoclastic giant cell-rich
tumour.[4] The term “aneurysmal bone cyst” is preferred
over “primary aneurysmal bone cyst”, while “ABC-like
change” is used instead of “secondary aneurysmal bone
cyst”, the latter commonly observed in giant cell tumours
of bone and chondroblastomas.
Reported cases of solid ABC (S-ABC) are primarily
found in the metaphyseal and diaphyseal regions of long
bones in children and young adults, typically during their
second or third decade of life.[5] [6] [7] [8] These cases usually present with an insidious onset of pain, swelling, or a
palpable mass. Although conventional ABC can involve
the flat bones of the pelvis, S-ABC arising from pelvic
bones in adults has not been reported, to the best of our
knowledge. ABC in the pelvis is often asymptomatic due
to its deep location, and by the time a patient develops
symptoms, the lesion has typically reached a significant
size. In our case, the lesion was an incidental finding
and relatively small without any complications; thus, the
patient remains asymptomatic.
S-ABC can be confidently differentiated from
conventional ABC on imaging. Although both can appear
as lytic lesions with sclerotic margins on radiography and
CT, conventional ABC typically exhibits an expansile
soap bubble appearance with internal septa, whereas
S-ABC often lacks septa and is non-expansile in up to
one third of cases.[5] Matrix mineralisation and periosteal reaction are generally absent in both forms.[9] MRI is
the modality of choice for aiding diagnosis. S-ABC is
predominantly solid with uniform contrast enhancement,
in contrast to the multiloculated cystic appearance and
peripheral and septal enhancement seen in conventional
ABC. Fluid-fluid levels can be observed in up to 70% of
conventional ABC cases but are not a consistent feature
in S-ABC.[7] Characteristically, S-ABC may be associated
with mild bone marrow and soft tissue oedema in up to
50% of cases.[8] The expansile nature of the lesion can
lead to marked thinning and focal disruption of the bony
cortex, as seen in our case during the initial presentation,
mimicking an aggressive bony lesion. Interestingly,
follow-up MRI after biopsy revealed multiple fluid-fluid
levels, while the previously noted bone marrow and
soft tissue oedema had resolved. Possible explanations
for these changes include interval haemorrhage within
the lesion due to the biopsy that could give rise to fluid-fluid levels. The bone marrow and soft tissue oedema
may have resulted from an inflammatory response to
the rapidly growing lesion in its initial phase, leading to
cortical disruption.[10]
The diagnostic challenge of S-ABC lies in distinguishing
it from ABC-like changes and other solid bone tumours.
The main differential diagnoses include giant cell
tumour of bone (GCTB), chondroblastoma, and primary
bone malignancies such as telangiectatic osteosarcoma.
GCTB typically occurs in a slightly older patient
population, demonstrates non-sclerotic margins, and
often extends to the subarticular surface of long bones.
Immunohistochemical staining for H3G34W and
H3K36M are specific markers that aid differentiation;
they are present in GCTB and chondroblastoma with
or without ABC-like changes, but are absent in ABC.[11]
In contrast, telangiectatic osteosarcoma is characterised
by geographic bone destruction, wide zones of
transition, and dense osteoid mineralisation. Extensive
soft tissue involvement and cortical destruction, along
with periosteal reaction on MRI, raise suspicion for a
malignant tumour rather than S-ABC.[12]
Biopsy and pathological examination are often necessary
when evaluating an aggressive-appearing lesion, and
radiological-pathological correlation plays a pivotal
role in reaching a diagnosis. S-ABC is characterised
by fibroblastic proliferation, osteoclast-like giant cells,
and focal osteoid production. In contrast to conventional
ABC, the blood-filled cystic spaces are present only
in small amounts, if at all. Histologically, S-ABC
resembles giant cell reparative granuloma and brown
tumours of hyperparathyroidism, as all three entities
exhibit giant cells, haemorrhagic areas, and reactive
osteoid formation.[8] Nonetheless, they lack the USP6
gene rearrangement.[13] Historically, the term S-ABC
was used interchangeably with giant cell reparative
granuloma; however, they are now regarded as distinct
entities, with the latter term reserved for lesions in the
gnathic location.[14] It is important to note that USP6 gene
rearrangement is not exclusive to ABCs; it has also been
identified in several other lesions, including nodular
fasciitis, myositis ossificans, fibro-osseous pseudotumour
of the digits, and cellular fibroma of the tendon sheath.
The diagnosis of S-ABC can be established through a
combination of compatible radiological features and
pathological examination.
CONCLUSION
S-ABC presents a unique diagnostic challenge due
to its similarities to other aggressive bone lesions.
Accurate diagnosis requires a comprehensive approach
that includes imaging, histological evaluation, and
identification of the USP6 gene rearrangement.
Radiologists should be aware of the distinct features of
S-ABC, particularly in contrast to conventional ABC and
other lesions such as giant cell tumour and telangiectatic
osteosarcoma.
REFERENCES
1. Nasri E, Reith JD. Aneurysmal bone cyst: a review. J Pathol Transl Med. 2023;57:81-7. Crossref
2. Sato K, Sugiura H, Yamamura S, Takahashi M, Nagasaka T, Fukatsu T. Solid variant of an aneurysmal bone cyst (giant cell reparative granuloma) of the 3rd lumbar vertebra. Nagoya J Med
Sci. 1996;59:159-65.
3. Cordier F, Creytens D. Unravelling the USP6 gene: an update. J Clin Pathol. 2023;76:573-7. Crossref
4. Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Bone:
an updated review. Adv Anat Pathol. 2021;28:119-38. Crossref
5. Ghosh A, Singh A, Yadav R, Khan SA, Kumar VS, Gamanagatti S.
Solid variant ABC of long tubular bones: a diagnostic conundrum
for the radiologist. Indian J Radiol Imaging. 2019;29:271-6. Crossref
6. Restrepo R, Zahrah D, Pelaez L, Temple HT, Murakami JW. Update
on aneurysmal bone cyst: pathophysiology, histology, imaging and
treatment. Pediatr Radiol. 2022;52:1601-14. Crossref
7. Al-Shamy G, Relyea K, Adesina A, Whitehead WE, Curry DJ,
Luerssen TG, et al. Solid variant of aneurysmal bone cyst of the
thoracic spine: a case report. J Med Case Rep. 2011;5:261. Crossref
8. Ilaslan H, Sundaram M, Unni KK. Solid variant of aneurysmal bone cysts in long tubular bones: giant cell reparative granuloma. AJR Am J Roentgenol. 2003;180:1681-7. Crossref
9. Yamaguchi T, Dorfman HD. Giant cell reparative granuloma:
a comparative clinicopathologic study of lesions in gnathic and
extragnathic sites. Int J Surg Pathol. 2001;9:189-200. Crossref
10. Mahnken AH, Nolte-Ernsting CC, Wildberger JE, Heussen N, Adam G, Wirtz DC, et al. Aneurysmal bone cyst: value of MR imaging and conventional radiography. Eur Radiol. 2003;13:1118-24. Crossref
11. Schaefer IM, Fletcher JA, Nielsen GP, Shih AR, Ferrone ML,
Hornick JL, et al. Immunohistochemistry for histone H3G34W
and H3K36M is highly specific for giant cell tumor of bone and
chondroblastoma, respectively, in FNA and core needle biopsy.
Cancer Cytopathol. 2018;126:552-66. Crossref
12. Zishan US, Pressney I, Khoo M, Saifuddin A. The differentiation
between aneurysmal bone cyst and telangiectatic osteosarcoma:
a clinical, radiographic and MRI study. Skeletal Radiol.
2020;49:1375-86. Crossref
13. Oliveira AM, Perez-Atayde AR, Inwards CY, Medeiros F, Derr V,
Hsi BL, et al. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am J Pathol. 2004;165:1773-80. Crossref
14. Lee JC, Huang HY. Soft tissue special issue: giant cell-rich lesions
of the head and neck region. Head Neck Pathol. 2020;14:97-108. Crossref