Intracranial Neuroendocrine Tumour of Unknown Origin Mimicking Neurocysticercosis: A Case Report
CASE REPORT
Hong Kong J Radiol 2025;28:Epub 10 December 2025
Intracranial Neuroendocrine Tumour of Unknown Origin
Mimicking Neurocysticercosis: A Case Report
CW Chan, KO Cheung, CY Cheung
Department of Radiology, North District Hospital, Hong Kong SAR, China
Correspondence: Dr CW Chan, Department of Radiology, North District Hospital, Hong Kong SAR, China. Email: ccw147@ha.org.hk
Submitted: 3 December 2024; Accepted: 11 April 2025. This version may differ from the final version when published in an issue.
Contributors: All authors designed the study. CWC acquired and analysed the data and drafted the manuscript. All authors critically revised
the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki and provided verbal consent for publication of this case
report, including the accompanying clinical images.
INTRODUCTION
Neuroendocrine tumours of the central nervous system
are relatively rare entities and most cases are metastases.
Primary intracranial neuroendocrine tumour is even rarer,
with fewer than a dozen cases reported worldwide.[1] [2] [3] [4] [5] [6] [7] [8]
Apart from a case report on a 5-year-old child,[9] all
reported primary cases have been in adults. The location
of lesions reported varies greatly. Reported extra-axial
locations include, but are not limited to, the
cerebellopontine angle, jugular foramen, cavernous
sinus, and skull base. Reported intra-axial locations include the left temporal and parietal lobes, as well as the left
cerebellum. We report a case of neuroendocrine tumour
of unknown origin with multiple intracranial metastases.
CASE PRESENTATION
A 56-year-old woman with good past health presented to
the accident and emergency department with dizziness
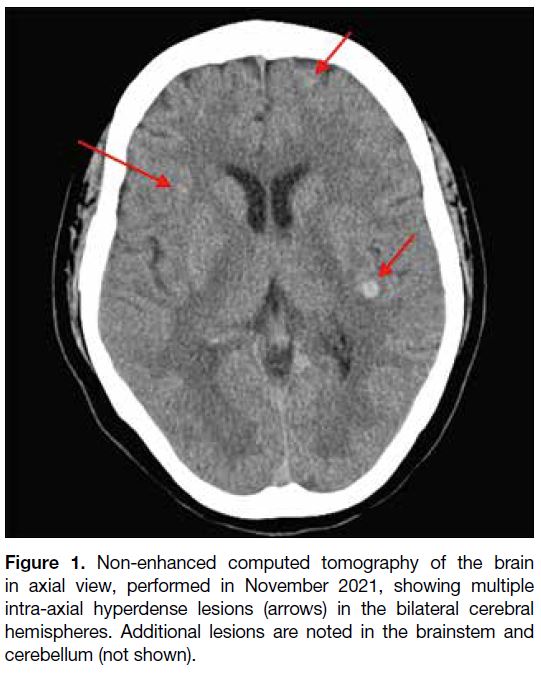
in October 2021. Initial computed tomography of the
brain revealed multiple hyperdense lesions scattered
across both cerebral hemispheres, the brainstem, and the
cerebellum (Figure 1). Whole-body positron emission
tomography–computed tomography (PET-CT) with 18F-fluorodeoxyglucose (18F-FDG) did not reveal any
primary malignancy elsewhere that could suggest brain
metastases. Contrast-enhanced magnetic resonance
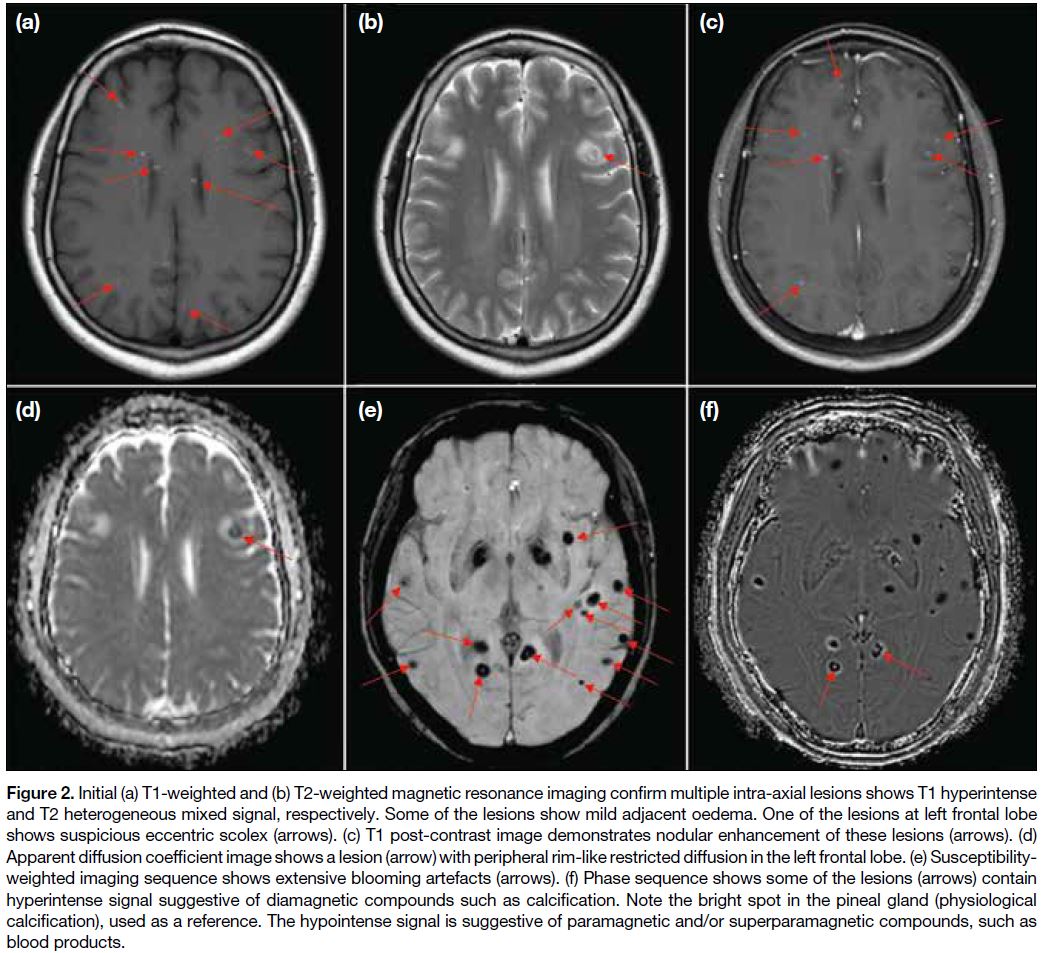
imaging (MRI) of the brain was performed to
characterise the intracranial lesions (Figure 2). The
corresponding cerebral, brainstem, and cerebellar lesions
showed T1 hyperintense and T2 heterogeneous mixed
signals. Most lesions showed susceptibility artefacts,
while some showed a signal on the phase sequence
characteristic of calcification. Overall features were
suggestive of concurrent haemorrhagic and calcified
lesions. Some lesions also showed eccentric nodular
enhancement. One 7-mm lesion in the left frontal lobe demonstrated restricted diffusion and a suspicious eccentric scolex (Figure 2b). In view of the previously
negative whole-body PET-CT, the possibility of central
nervous system infection with neurocysticercosis in
different stages was considered a likely possibility. A
differential diagnosis of haemorrhagic/calcified brain
metastases appeared less likely. Serology testing for
Taenia solium was negative, but given the radiological
appearance of neurocysticercosis, the patient was
prescribed a course of albendazole and praziquantel, as
well as dexamethasone to minimise cerebral oedema.
Figure 1. Non-enhanced computed tomography of the brain
in axial view, performed in November 2021, showing multiple
intra-axial hyperdense lesions (arrows) in the bilateral cerebral
hemispheres. Additional lesions are noted in the brainstem and
cerebellum (not shown).
Figure 2. Initial (a) T1-weighted and (b) T2-weighted magnetic resonance imaging confirm multiple intra-axial lesions shows T1 hyperintense
and T2 heterogeneous mixed signal, respectively. Some of the lesions show mild adjacent oedema. One of the lesions at left frontal lobe
shows suspicious eccentric scolex (arrows). (c) T1 post-contrast image demonstrates nodular enhancement of these lesions (arrows). (d)
Apparent diffusion coefficient image shows a lesion (arrow) with peripheral rim-like restricted diffusion in the left frontal lobe. (e) Susceptibility-weighted
imaging sequence shows extensive blooming artefacts (arrows). (f) Phase sequence shows some of the lesions (arrows) contain
hyperintense signal suggestive of diamagnetic compounds such as calcification. Note the bright spot in the pineal gland (physiological
calcification), used as a reference. The hypointense signal is suggestive of paramagnetic and/or superparamagnetic compounds, such as
blood products.
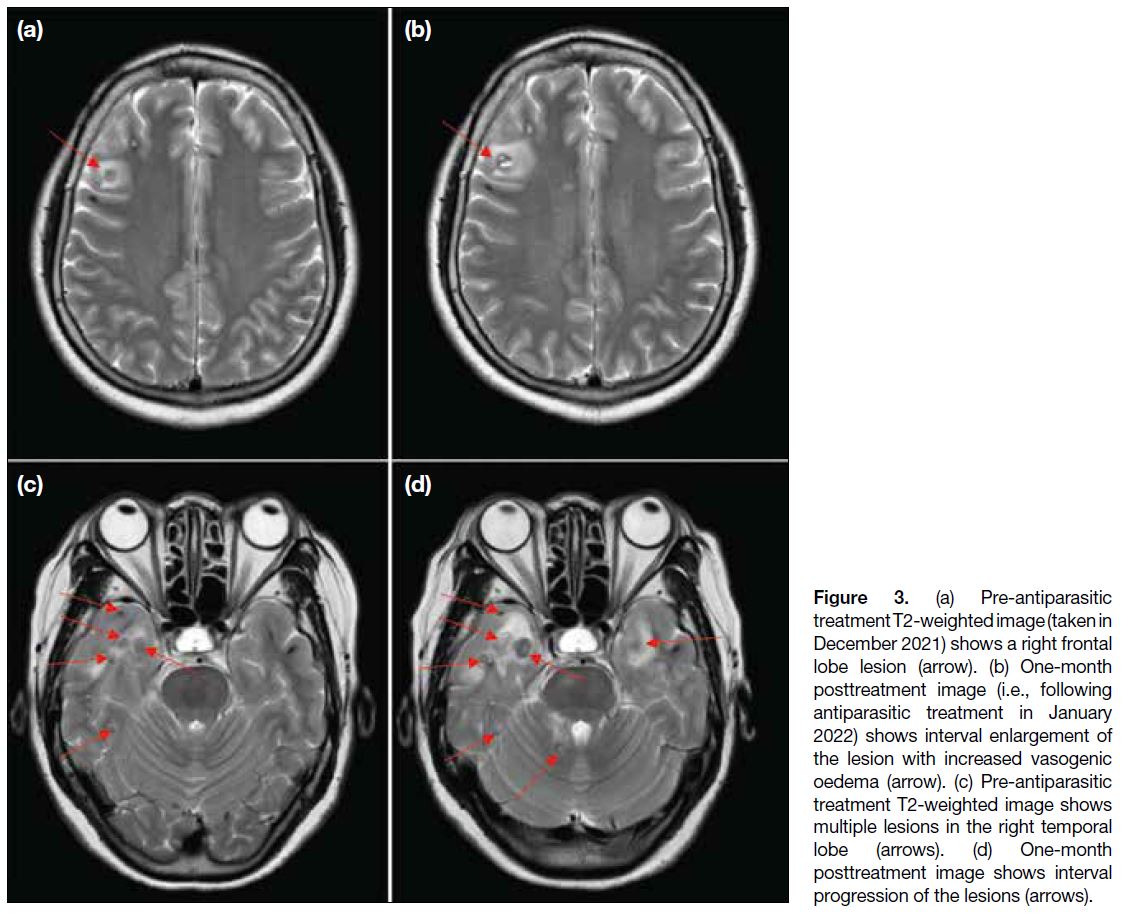
Multiple follow-up MRI scans of the brain were
performed. Initially, at 1-month post-treatment, some
lesions (particularly those at the frontal and temporal lobes) showed interval enlargement with an increase in
perilesional vasogenic oedema (Figure 3). These findings
were thought to be attributable to posttreatment change.
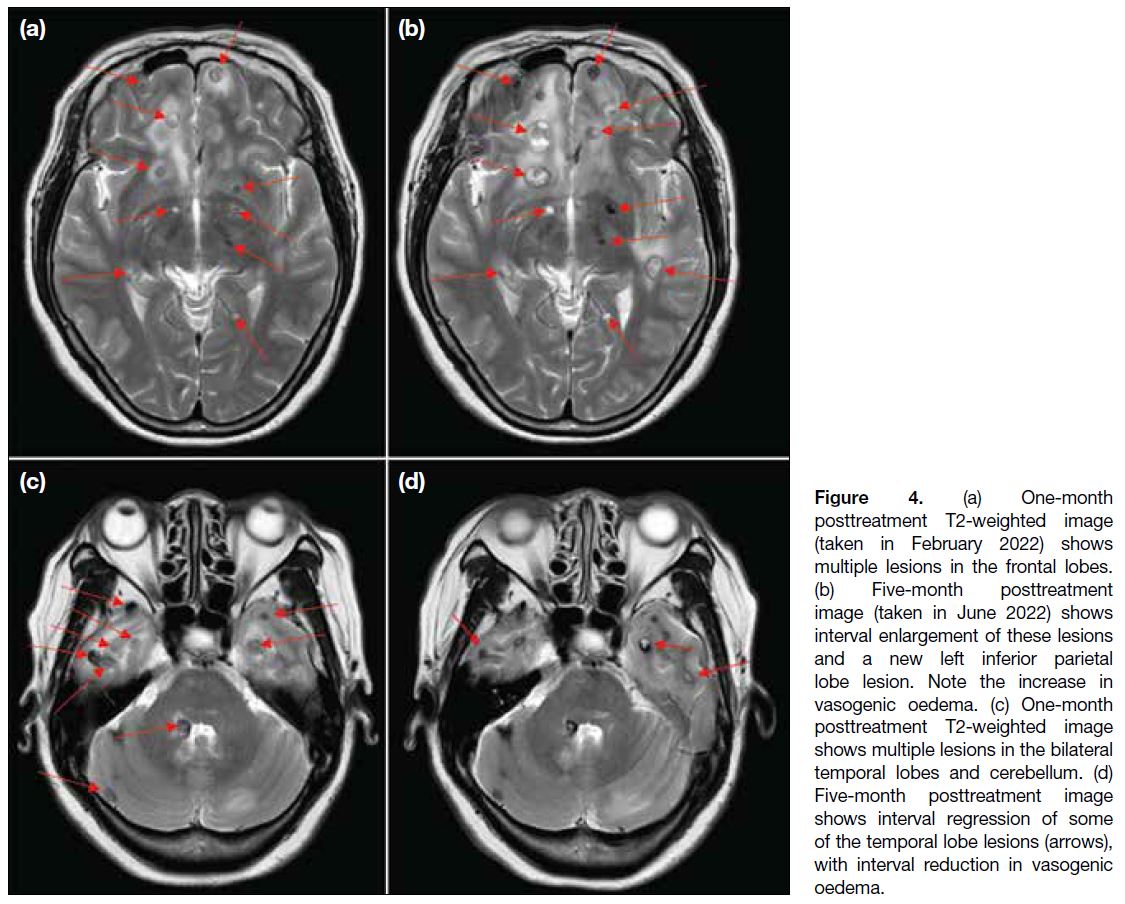
A scan at 5 months posttreatment revealed continued progression of some lesions in the bilateral frontal and left inferior parietal lobes (Figure 4a and b), while some lesions in the bilateral temporal lobes had regressed (Figure 4c and d). The overall picture favoured a mixed treatment response.
Figure 3. (a) Pre-antiparasitic
treatment T2-weighted image (taken in
December 2021) shows a right frontal
lobe lesion (arrow). (b) One-month
posttreatment image (i.e., following
antiparasitic treatment in January
2022) shows interval enlargement of
the lesion with increased vasogenic
oedema (arrow). (c) Pre-antiparasitic
treatment T2-weighted image shows
multiple lesions in the right temporal
lobe (arrows). (d) One-month
posttreatment image shows interval
progression of the lesions (arrows).
Figure 4. (a) One-month
posttreatment T2-weighted image
(taken in February 2022) shows
multiple lesions in the frontal lobes.
(b) Five-month posttreatment
image (taken in June 2022) shows
interval enlargement of these lesions
and a new left inferior parietal
lobe lesion. Note the increase in
vasogenic oedema. (c) One-month
posttreatment T2-weighted image
shows multiple lesions in the bilateral
temporal lobes and cerebellum. (d)
Five-month posttreatment image
shows interval regression of some
of the temporal lobe lesions (arrows),
with interval reduction in vasogenic
oedema.
A further course of antiparasitic treatment was given,
assuming the infection was unresolved. Nonetheless,
follow-up scan at 16 months after initiation of antiparasitic
treatment showed not only persistent lesions, but interval
enlargement of some (the largest at the left cerebellar
hemisphere; Figure 5), with developing obstructive
hydrocephalus. In view of the patient’s worsening
symptoms of increased intracranial pressure (headache, dizziness and vomiting), as well as imaging findings,
the neurosurgical team intervened and left posterior
craniotomy was performed for decompression and to
excise the left cerebellar lesion. An external ventricular
drain was placed. Intraoperative findings noted a large
intra-axial tumour at the left cerebellar hemisphere,
likely malignant. Pathology confirmed a grade 3
neuroendocrine tumour, with additional comment that
a metastatic lesion was likely. A repeated whole-body
PET-CT with gallium-68-DOTA-tyr3-octreotate (Ga-68-DOTATATE) showed multiple hypermetabolic nodules
in the brain suggestive of known neuroendocrine tumour
(Figure 6), but still no obvious location for a primary
malignancy. A preliminary diagnosis was reached of
neuroendocrine tumour of unknown origin, with possible
primary within the brain. Postoperatively, the patient
underwent further follow-up MRI scans that revealed
new suspicious drop metastasis at the C4 level, as well as significant progression of brain metastases and
worsening vasogenic oedema (Figure 7). The patient
was followed up by the neurosurgery and oncology
teams and underwent radiotherapy of the whole brain
and the cervical spinal cord as palliative care. At 35
months after the initial presentation, the patient died due
to a complication of pneumonia.
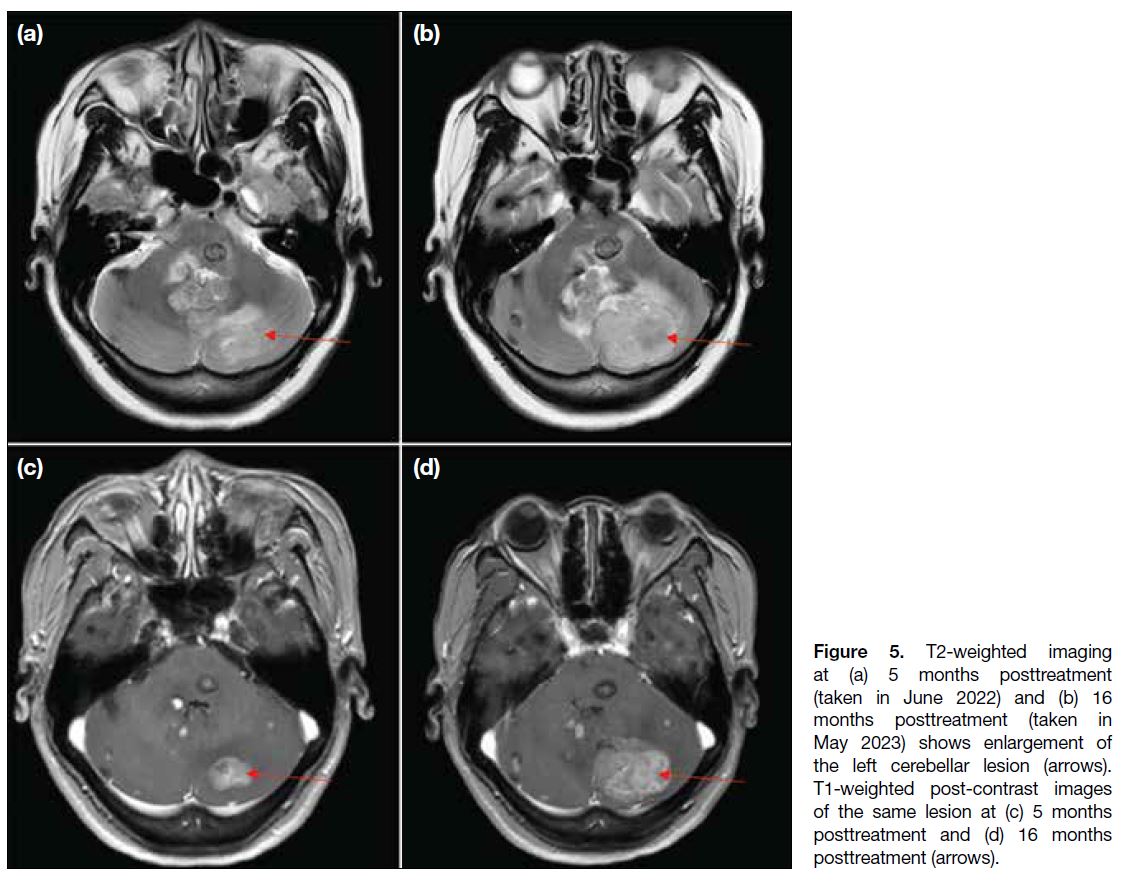
Figure 5. T2-weighted imaging
at (a) 5 months posttreatment
(taken in June 2022) and (b) 16
months posttreatment (taken in
May 2023) shows enlargement of
the left cerebellar lesion (arrows).
T1-weighted post-contrast images
of the same lesion at (c) 5 months
posttreatment and (d) 16 months
posttreatment (arrows).
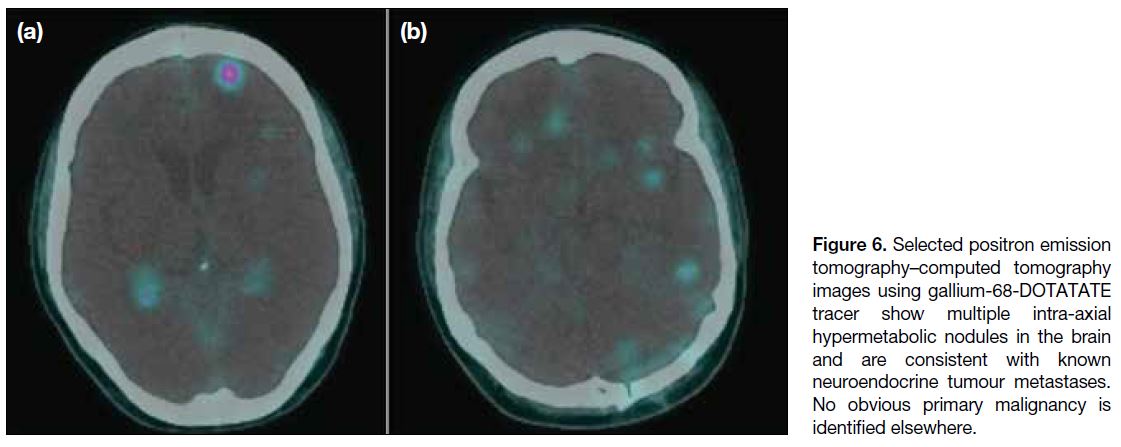
Figure 6. Selected positron emission
tomography–computed tomography
images using gallium-68-DOTATATE
tracer show multiple intra-axial
hypermetabolic nodules in the brain
and are consistent with known
neuroendocrine tumour metastases.
No obvious primary malignancy is
identified elsewhere.
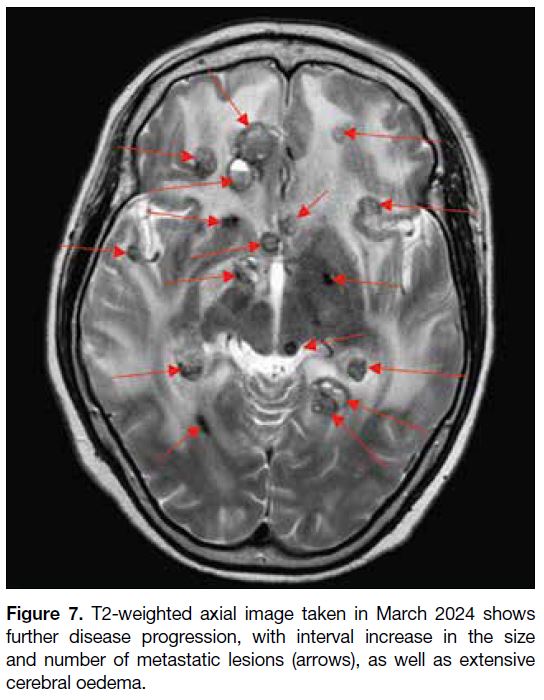
Figure 7. T2-weighted axial image taken in March 2024 shows
further disease progression, with interval increase in the size
and number of metastatic lesions (arrows), as well as extensive
cerebral oedema.
DISCUSSION
Neurocysticercosis and neuroendocrine tumour of the
brain are two distinct entities that require very
different treatment approaches. The patient’s presenting
signs and symptoms (such as headache, dizziness and
seizure) are often non-specific. Serology testing for
Taenia solium, while specific, is often not sensitive. A
negative serology test does not exclude the diagnosis
of neurocysticercosis; hence, it was reasonable for our patient to undergo a trial of antiparasitic treatment based
on radiological appearance alone.
Imaging plays an important role in guiding the diagnosis
as well as treatment in such difficult cases. Nonetheless,
as with our case, imaging also has its limitations and can
be misguided by disease mimics.
On MRI, neurocysticercosis has varied radiological
appearances depending on its four main stages.[10] [11]
During the vesicular stage, cysts with cerebrospinal
fluid (CSF) intensity are often seen, sometimes with an
eccentric scolex that may show enhancement. Typically,
no surrounding vasogenic oedema is seen at this stage.
Intraventricular cysts may be difficult to visualise, and
heavily T2-weighted sequences such as FIESTA (fast
imaging employing steady-state acquisition) may help delineate the walls and scolex of neurocysticercosis. In
addition, the cystic content may show a slightly lower
signal compared with CSF, making them stand out.[12] For
our case, the FIESTA sequence was not performed due
to limited resources.
During the colloidal vesicular stage, cysts will often
contain increased proteinaceous content, leading to T1
and fluid-attenuated inversion recovery hyperintense
signal relative to CSF. Thickening and enhancement of
the cyst wall, as well as surrounding oedema, may be
seen. Some lesions may also show restricted diffusion,[11]
as in our case, which further complicates the clinical picture.
During the granular nodular stage, the cystic component
will resolve, becoming a small enhancing nodule.
Contrast enhancement and perilesional oedema will
gradually decrease and eventually resolve in the final
nodular calcified stage, where calcified nodules are seen. Neuroendocrine tumour of the brain, whether primary
or secondary, can also have variable appearances
mimicking other diseases. Among the reported primary
cases, MRI appearances ranged from a
solid enhancing mass to a cystic mass with a peripheral
enhancing component.[1] [2] [3] [4] [5] [6] [7] [8] [9]
Spontaneous regression of up to one quarter of
neuroendocrine tumours has also been reported, albeit
most were extracranial in location, possibly due to host
immune response against neoantigens expressed by the
tumour.[13] This further increases diagnostic confusion, as
in our patient, and led us to interpret the regression of
lesions as a partial response to antiparasitic treatment.
Other imaging modalities such as PET scan may
offer more diagnostic clues, but 18F-FDG, which is
the most common tracer, may not show uptake in well-differentiated neuroendocrine tumours. On the
contrary, Ga-68-DOTATATE has a high sensitivity and
specificity in the detection of neuroendocrine tumours.[14]
Contrary to 18F-FDG which targets glucose metabolism,
Ga-68-DOTATATE targets somatostatin receptors that
are usually overexpressed by neuroendocrine tumours.
Nonetheless, this tracer is not yet widely available in our
region.
With hindsight, there are lessons to be learnt from
our patient and improvements to be made, especially
in her management. There was an 11-month period
(between 5 and 16 months posttreatment) with
no imaging follow-up or further workup. There were
already significantly enlarging lesions on the 5-month
posttreatment scan, and although present, regression of
the temporal lesions was subtle. More aggressive follow-up
imaging (e.g., within a few months) would have been
appropriate.
Furthermore, brain parenchymal haemorrhage, which
was already present on her initial MRI scan, is an
uncommon finding in neurocysticercosis. Alternative
differential diagnoses should have been considered,
especially in view of the suboptimal radiological
response to antiparasitic treatment. Given the vital
location of the enlarging lesions, further investigations
such as brain biopsy should also have been considered
and offered at an earlier stage.
Although treatment trials with antiparasitic drugs and
interval follow-up scans may provide a general idea of
the course of the disease, histological diagnosis including
excisional biopsy may be the only means by which to
confirm a diagnosis.
CONCLUSION
Neurocysticercosis in our region is uncommon, and
neuroendocrine tumour of the brain is even rarer. We
encountered an atypical presentation of a neuroendocrine
tumour of the brain mimicking neurocysticercosis. A
multidisciplinary approach involving the infectious diseases team, as well as neurosurgical and oncological
specialists, is necessary to reach definitive diagnosis.
REFERENCES
1. Caro-Osorio E, Perez-Ruano LA, Martinez HR, Rodriguez-Armendariz AG, Lopez-Sotomayor DM. Primary neuroendocrine
carcinoma of the cerebellopontine angle: a case report and literature
review. Cureus. 2022;14:e27564. Crossref
2. Porter DG, Chakrabarty A, McEvoy A, Bradford R. Intracranial
carcinoid without evidence of extracranial disease. Neuropathol
Appl Neurobiol. 2000;26:298-300. Crossref
3. Deshaies EM, Adamo MA, Qian J, DiRisio DA. A carcinoid tumor
mimicking an isolated intracranial meningioma. Case report. J
Neurosurg. 2004;101:858-60. Crossref
4. Ibrahim M, Yousef M, Bohnen N, Eisbruch A, Parmar H. Primary
carcinoid tumor of the skull base: case report and review of the
literature. J Neuroimaging. 2010;20:390-2. Crossref
5. Hakar M, Chandler JP, Bigio EH, Mao Q. Neuroendocrine
carcinoma of the pineal parenchyma. The first reported case. J Clin
Neurosci. 2017;35:68-70. Crossref
6. Liu H, Wang H, Qi X, Yu C. Primary intracranial neuroendocrine
tumor: two case reports. World J Surg Oncol. 2016;14:138. Crossref
7. Reed CT, Duma N, Halfdanarson T, Buckner J. Primary
neuroendocrine carcinoma of the brain. BMJ Case Rep. 2019;12:e230582. Crossref
8. Tamura R, Kuroshima Y, Nakamura Y. Primary neuroendocrine
tumor in brain. Case Rep Neurol Med. 2014;2014:295253. Crossref
9. Stepien N, Haberler C, Theurer S, Schmook M, Lütgendorf-Caucig
C, Müllauer L, et al. Unique finding of a primary central nervous
system neuroendocrine carcinoma in a 5-year-old child: a case
report. Front Neurosci. 2022;16:810645. Crossref
10. Teitelbaum GP, Otto RJ, Lin M, Watanabe AT, Stull MA, Manz HJ, et al. MR imaging of neurocysticercosis. AJR Am J
Roentgenol. 1989;153:857-66. Crossref
11. Santos GT, Leite CC, Machado LR, McKinney AM, Lucato LT.
Reduced diffusion in neurocysticercosis: circumstances of
appearance and possible natural history implications. AJNR Am J
Neuroradiol. 2013;34:310-6. Crossref
12. Neyaz Z, Patwari SS, Paliwal VK. Role of FIESTA and SWAN
sequences in diagnosis of intraventricular neurocysticercosis.
Neurol India. 2012;60:646-7. Crossref
13. Amoroso V, Agazzi GM, Roca E, Fazio N, Mosca A, Ravanelli M, et al. Regression of advanced neuroendocrine tumors among
patients receiving placebo. Endocr Relat Cancer. 2017;24:L13-6. Crossref
14. Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role
of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in
patients with neuroendocrine tumors: a meta-analysis. Acta Radiol
2014;55:389-98. Crossref